Ionic Formulas Rule of Eight ionic compounds metal

- Slides: 5

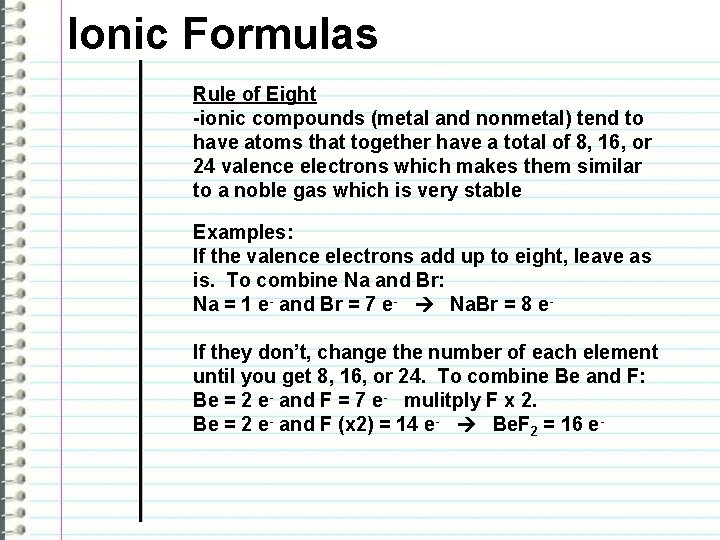
Ionic Formulas Rule of Eight -ionic compounds (metal and nonmetal) tend to have atoms that together have a total of 8, 16, or 24 valence electrons which makes them similar to a noble gas which is very stable Examples: If the valence electrons add up to eight, leave as is. To combine Na and Br: Na = 1 e- and Br = 7 e- Na. Br = 8 e. If they don’t, change the number of each element until you get 8, 16, or 24. To combine Be and F: Be = 2 e- and F = 7 e- mulitply F x 2. Be = 2 e- and F (x 2) = 14 e- Be. F 2 = 16 e-

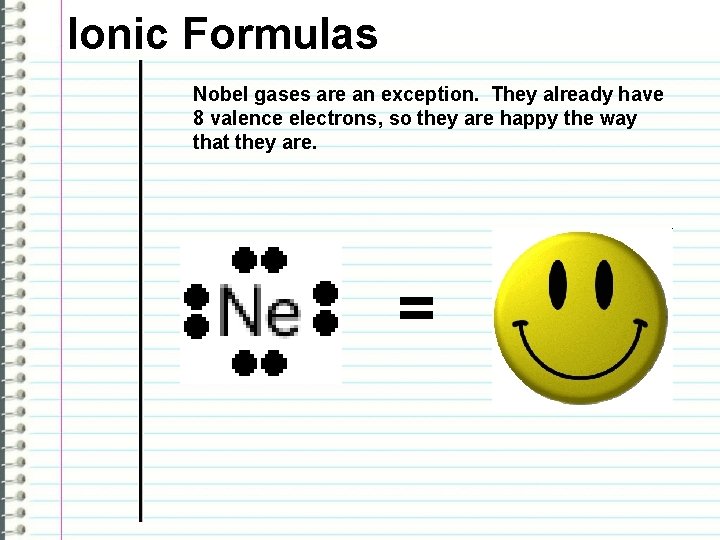
Ionic Formulas Nobel gases are an exception. They already have 8 valence electrons, so they are happy the way that they are. =

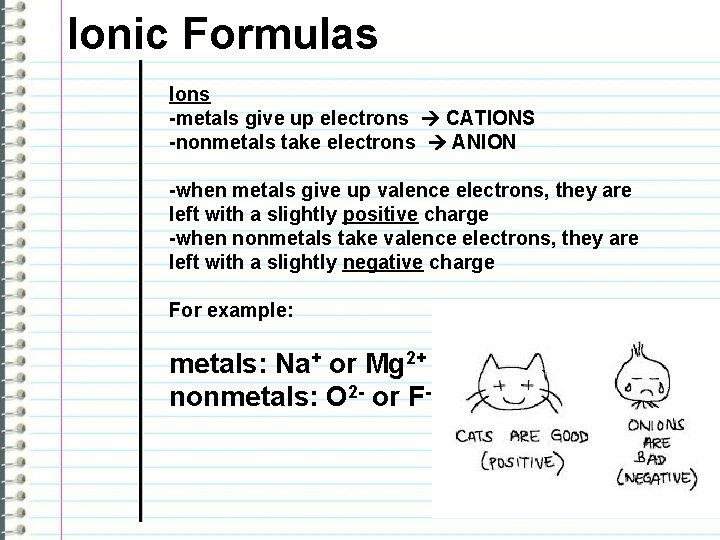
Ionic Formulas Ions -metals give up electrons CATIONS -nonmetals take electrons ANION -when metals give up valence electrons, they are left with a slightly positive charge -when nonmetals take valence electrons, they are left with a slightly negative charge For example: metals: Na+ or Mg 2+ nonmetals: O 2 - or F-

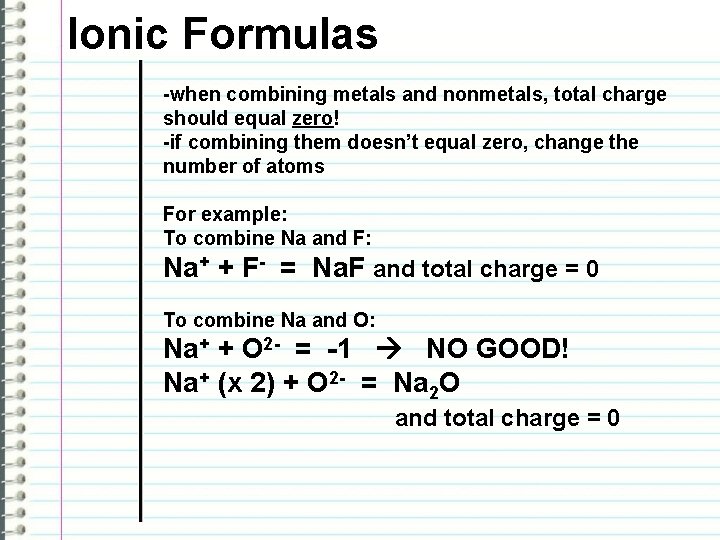
Ionic Formulas -when combining metals and nonmetals, total charge should equal zero! -if combining them doesn’t equal zero, change the number of atoms For example: To combine Na and F: Na+ + F- = Na. F and total charge = 0 To combine Na and O: Na+ + O 2 - = -1 NO GOOD! Na+ (x 2) + O 2 - = Na 2 O and total charge = 0

Ions and Ionic Bond Practice 1. Write down the name of two ions/polyatomic ions that have a 1+ charge. How many electrons have they gained or lost? 2. Write down the name of two ions/polyatomic ions that have a 2 - charge. How many electrons have they gained or lost? 3. Write down the name of two elements that will always have a 0 charge. How many electrons do they gain or lose? Put together the following monatomic ions into compounds. 4. Ca and O 5. Ba and F 6. K and N 7. Mg and P 8. Li and Cl