Ionic Covalent Hydrogen Bonds Bonds Ionic Covalent Transfer
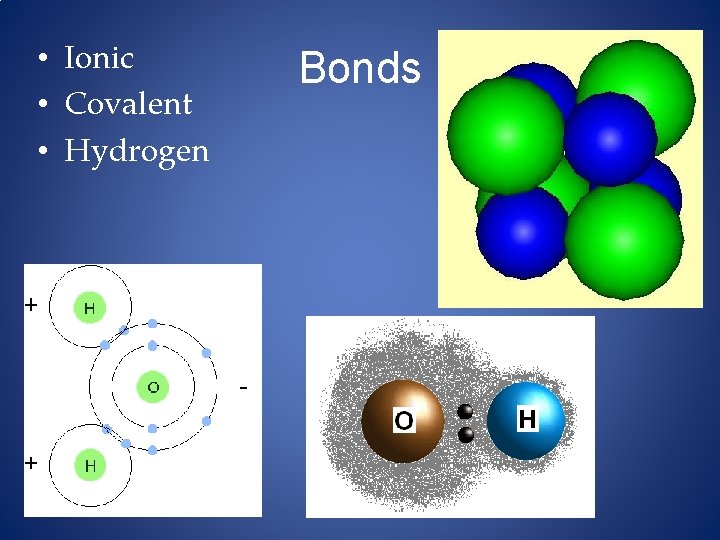
• Ionic • Covalent • Hydrogen Bonds
Bonds Ionic Covalent • Transfer electrons • Form between ions (+/-) • Share electrons • Weaker than ionic • 2 types – Polar – Nonpolar
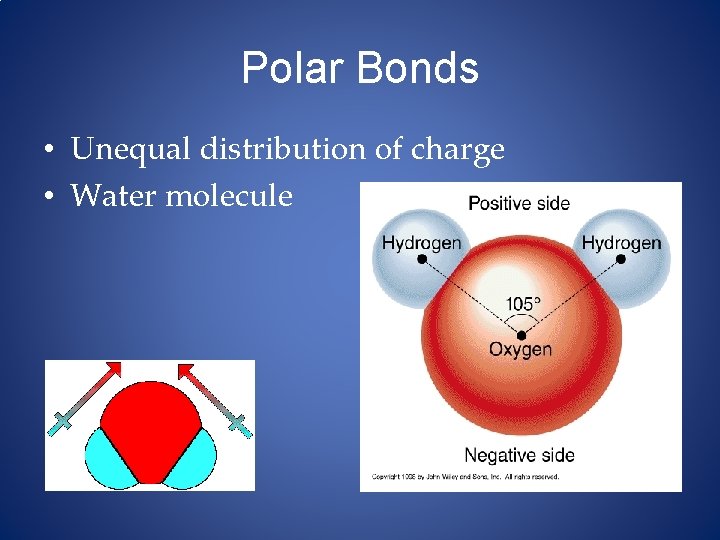
Polar Bonds • Unequal distribution of charge • Water molecule

Nonpolar • Equal sharing of electrons
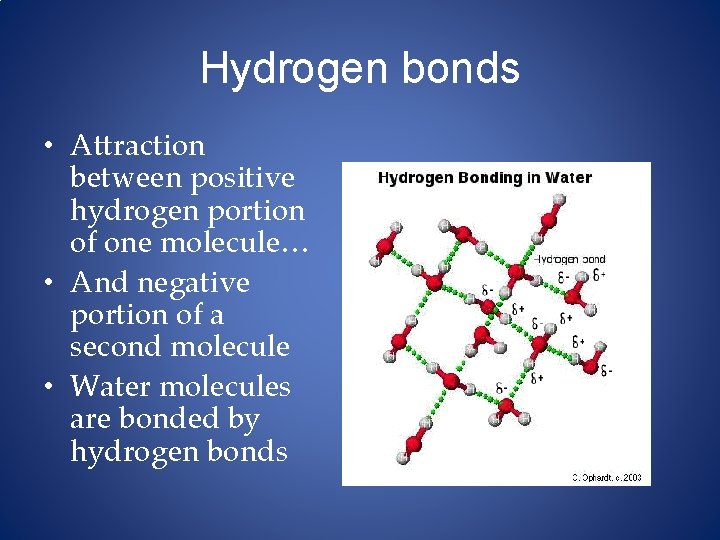
Hydrogen bonds • Attraction between positive hydrogen portion of one molecule… • And negative portion of a second molecule • Water molecules are bonded by hydrogen bonds

Properties of Water • Cohesion – Attractive force – Holds together – Due to H-bonding – ex: water sticking to other water molecules-meniscus

Properties of Water • Surface tension • Related to cohesion • Due to Hbonds • Cause water to be pulled down into liquid • “skin” on top of the water • Water striders • http: //www. y outube. com/w atch? v=KSLU wm. JOo_M

Properties of Water • Adhesion – Attractive force between two different surfaces – Ex: Water molecules and glass molecules

Properties of Water • Capillary action • Raising of liquid • Glass tube, water will rise automatically

Acids and Bases

Acids: • Taste sour • Contains H+, (the more H+, the stronger the acid) • Very reactive

Properties of Bases: • Taste bitter • Contains OH- (the more OH-, the stronger the base) • Slippery when wet

Acids and Bases • If the number of H+ ions in a solution is greater than number of OH- ions than the solution is an acid • If the OH- ions are greater than the H+ ions than the solution is a base
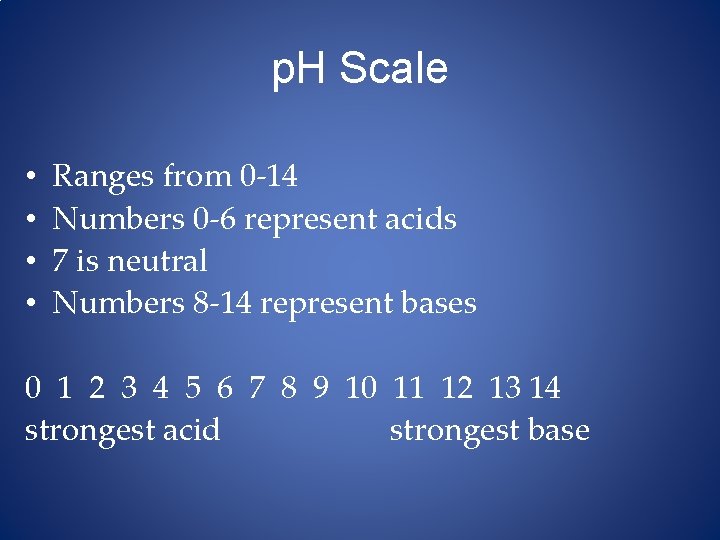
p. H Scale • • Ranges from 0 -14 Numbers 0 -6 represent acids 7 is neutral Numbers 8 -14 represent bases 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 strongest acid strongest base
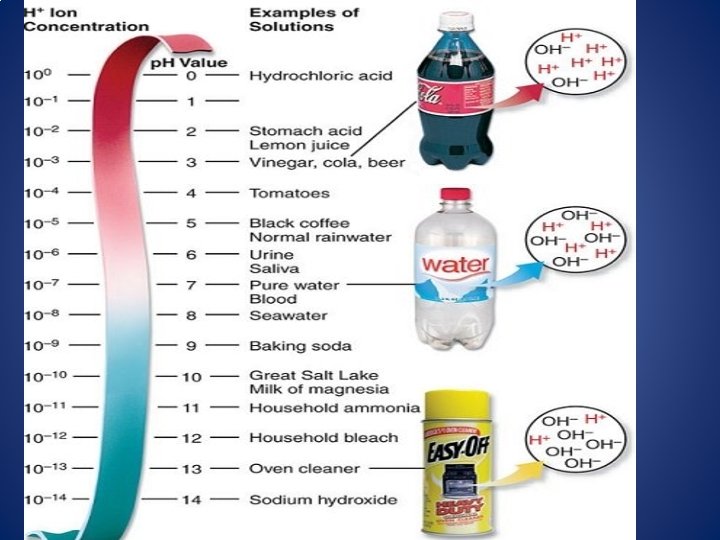
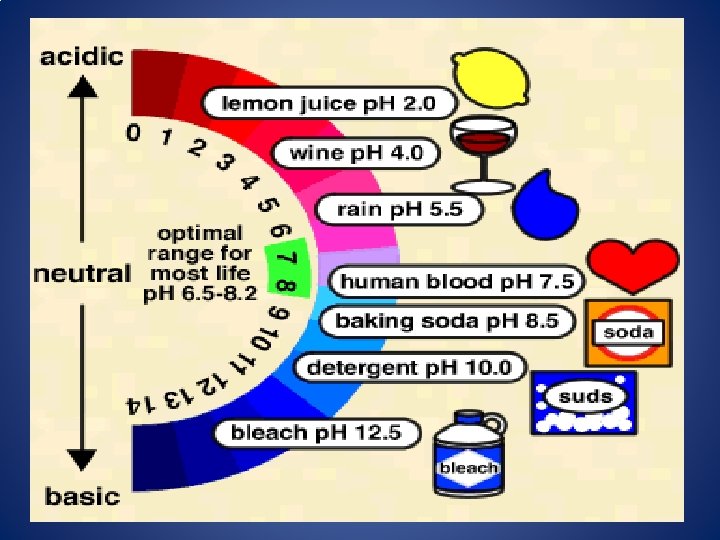
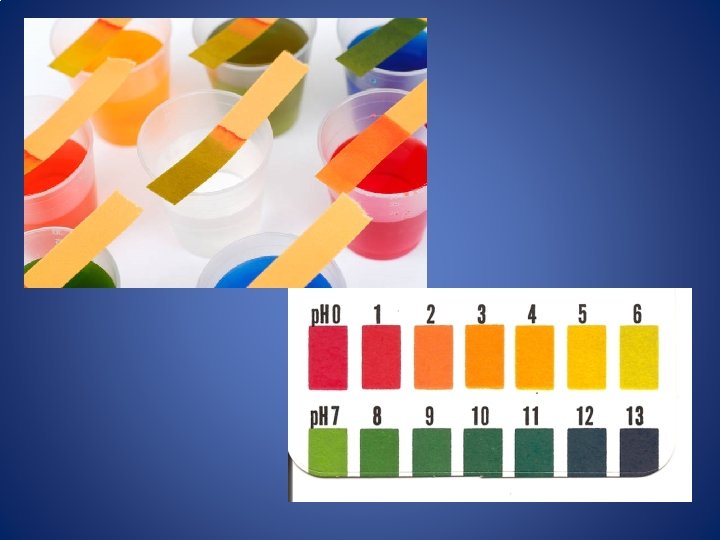

p. H Scale As you move from p. H 0 to p. H 14, the concentration of H+ DECREASES (10 times each number) As you move from p. H 0 to p. H 14, the concentration of OH- INCREASES (10 times each number) Ex: A substance with p. H 3 is 100 times stronger acid than a substance with p. H 5. A substance with p. H 8 is 100 times weaker base than a substance with p. H 10.

Buffer • Neutralize acids or bases • Keep the p. H from large changes
- Slides: 19