IONIC COVALENT A positive or a negative number
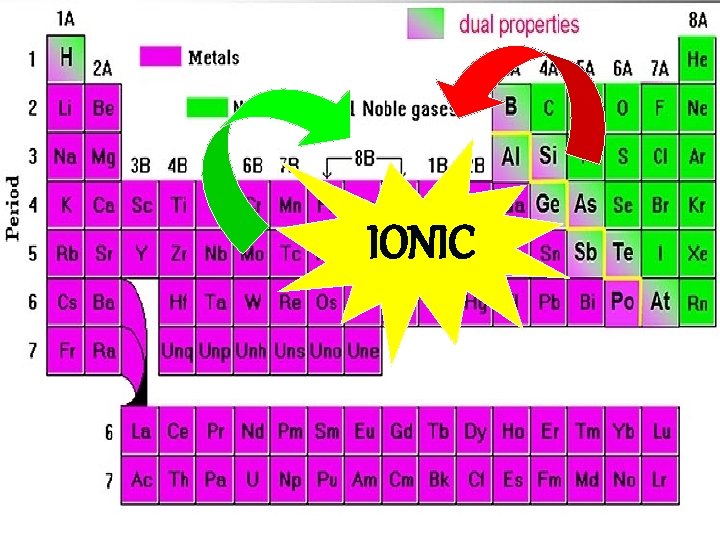
IONIC
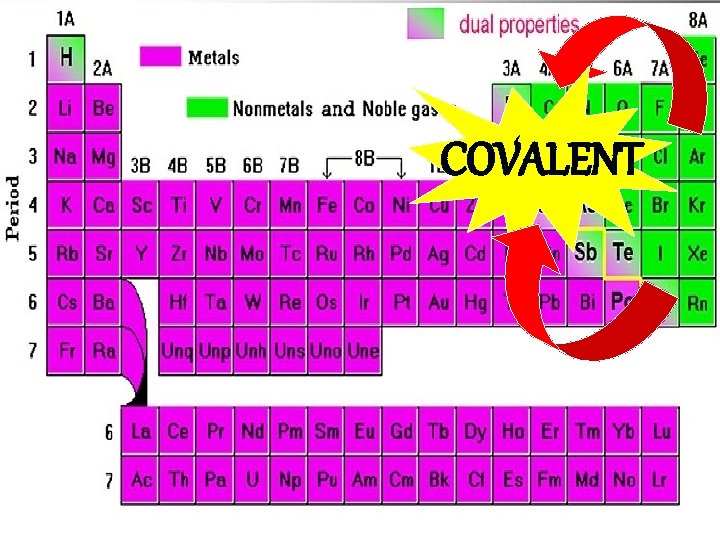
COVALENT

A positive or a negative number assigned to a combined atom according to a set of arbitrary (agreed upon) rules.


1. The oxidation number of an atom of a free element is zero. This is true even for polyatomic (many atoms) elements. Ex. 0 Na 0 Al 0 O 2 0 S 8

2. The oxidation number of a monoatomic (one) ion is equal to its charge. Ex. +1 Na +1 +2 Mg +2 -1 Cl -

3. The oxidation number of oxygen in a compound is usually -2. However, in peroxides (O 2 in compound), oxygen has a lower oxidation state, -1. When bound to fluorine, the oxidation number of oxygen is +2. (Ex) +1 -2 +1 -1 H 2 O 2 +2 -1 O F 2

4. The oxidation number of hydrogen in a compound is usually +1 when combined with a non-metal. In the presence of a metal, its oxidation number is -1. (Ex) +1 -1 -3 +1 H Cl N H 3 +1 -1 Li H +2 -1 Mg H 2

5. In combination of non-metals, the oxidation number of the less electronegative elements is positive and the more electronegative element, negative. (Ex) +1 -2 +1 -1 +5 -2 H 2 O HF P 2 O 5

6. The sum of all oxidation numbers of all the atoms in a neutral compound is zero. (Ex) +1 -1 Na Cl 1(+1) +1 (-1) = 0 +1 -2 K 2 S 2(+1) + 1(-2) = 0

7. The sum of the oxidation numbers of all atoms in the formula of a polyatomic (many atoms) ion is equal to its charge. (Ex) +5 -2 N O 3 1(+5) + 3(-2) = -1 -2 +1 O H 1(-2) + 1(+1) = -1
- Slides: 11