Ionic Compounds Writing Formulas Empirical Formulas smallest wholenumber

Ionic Compounds: Writing Formulas

Empirical Formulas = smallest whole-number ratio of elements in compound • Ionic compounds only have empirical formulas
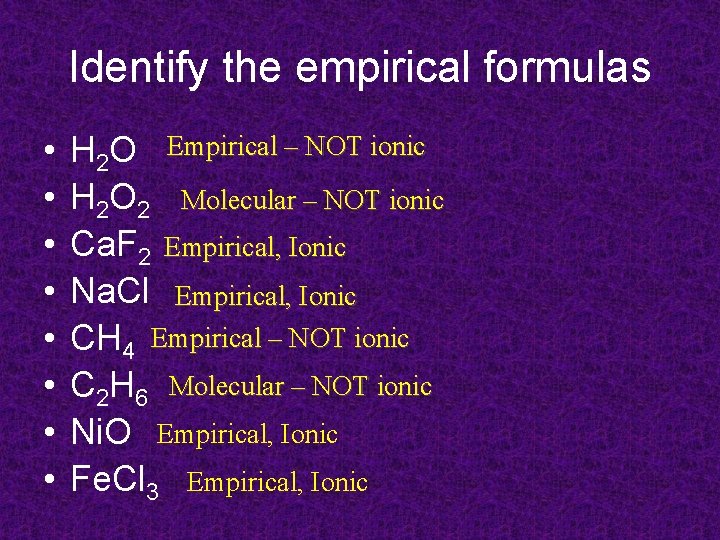
Identify the empirical formulas • • H 2 O Empirical – NOT ionic H 2 O 2 Molecular – NOT ionic Ca. F 2 Empirical, Ionic Na. Cl Empirical, Ionic CH 4 Empirical – NOT ionic C 2 H 6 Molecular – NOT ionic Ni. O Empirical, Ionic Fe. Cl 3 Empirical, Ionic
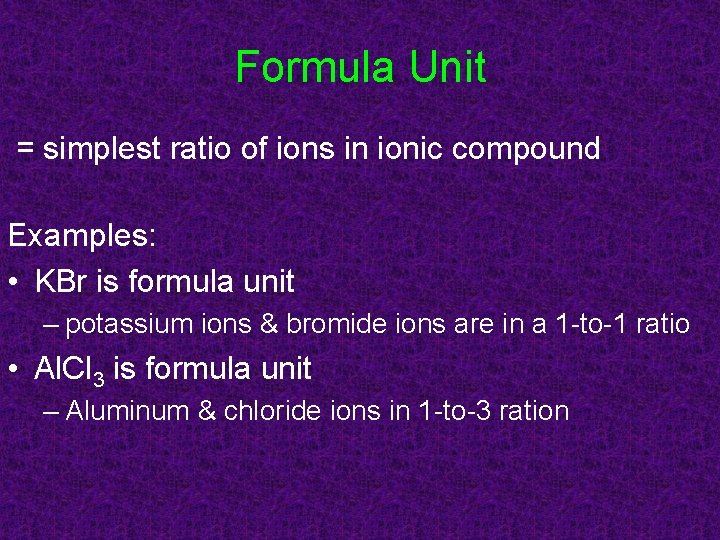
Formula Unit = simplest ratio of ions in ionic compound Examples: • KBr is formula unit – potassium ions & bromide ions are in a 1 -to-1 ratio • Al. Cl 3 is formula unit – Aluminum & chloride ions in 1 -to-3 ration

Binary Ionic Compounds • Composed of two different elements – Positive monatomic metal ion – Negative monatomic nonmetal ion Note: A binary compound may contain more than 2 ions but only 2 kinds of ions like Al 2 O 3
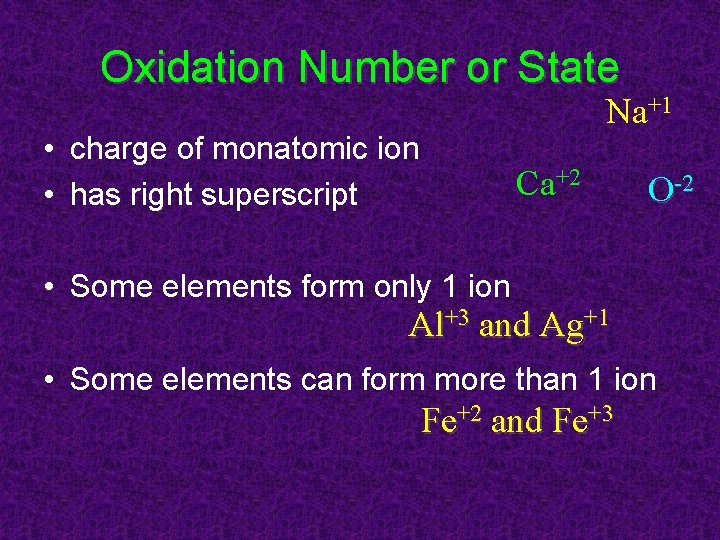
Oxidation Number or State Na+1 • charge of monatomic ion • has right superscript Ca+2 O-2 • Some elements form only 1 ion Al+3 and Ag+1 • Some elements can form more than 1 ion Fe+2 and Fe+3

Oxidation States • • Group 1 ions always +1 Group 2 ions always +2 MOST group 13 ions are +3 Groups 14, 15, 16, and 17 have multiple oxidation states – both (+) and (-) • Transition metals (Group B) can have more than 1 oxidation state – but are always (+)

Writing Formulas for Binary Ionic Compounds • first rule in writing formulas for ionic compounds is POSITIVE ION FIRST But how do you get the subscripts? start by writing formulas from the ions

• Compounds are electrically neutral – Sum of all charges in compound should be 0 – (+) and (-) charges must = each other
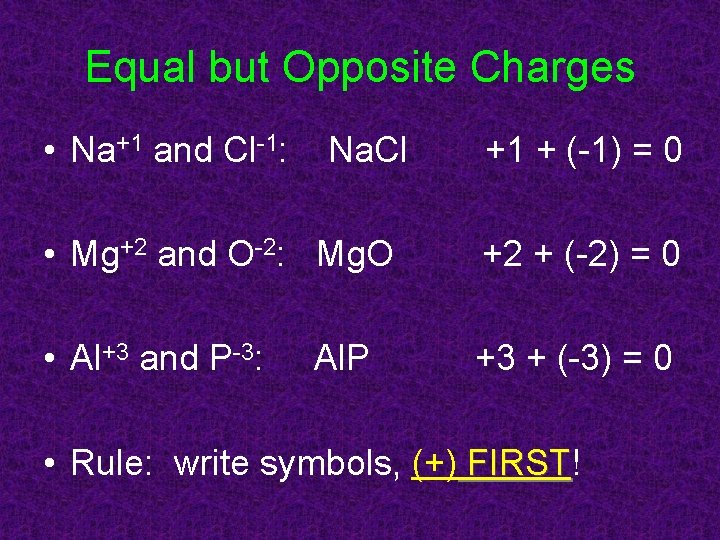
Equal but Opposite Charges • Na+1 and Cl-1: Na. Cl +1 + (-1) = 0 • Mg+2 and O-2: Mg. O +2 + (-2) = 0 • Al+3 and P-3: +3 + (-3) = 0 Al. P • Rule: write symbols, (+) FIRST! FIRST
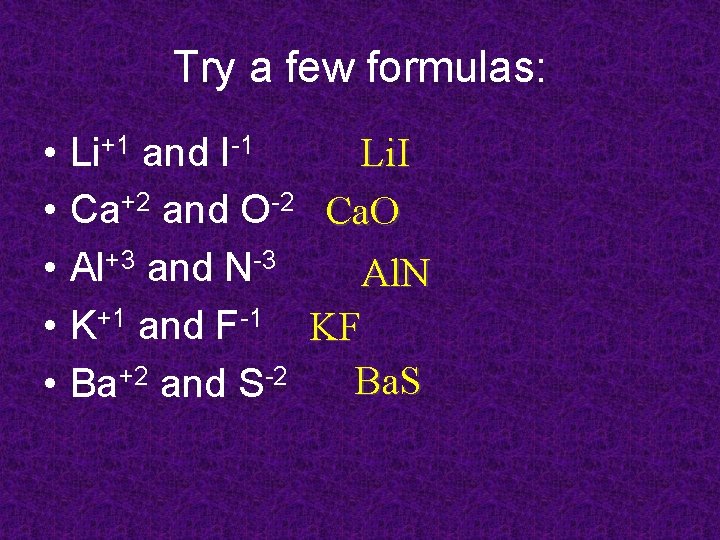
Try a few formulas: • • • Li. I Li+1 and I-1 Ca+2 and O-2 Ca. O Al+3 and N-3 Al. N K+1 and F-1 KF Ba. S Ba+2 and S-2

Criss-Cross Method If charges are not equal but opposite: • Mg+2 and Cl-1, CROSS and DROP! (# only, forget signs!) • Mg 1 Cl 2 but if subscript is 1, forget it! • Mg. Cl 2 means 1 Mg+2 and 2 Cl-1

Check the Math • Mg. Cl 2 means 1 Mg+2 and 2 Cl-1 (1)(+2) + (2)(-1) = +2 + -2 = 0 • charges MUST add up to zero!
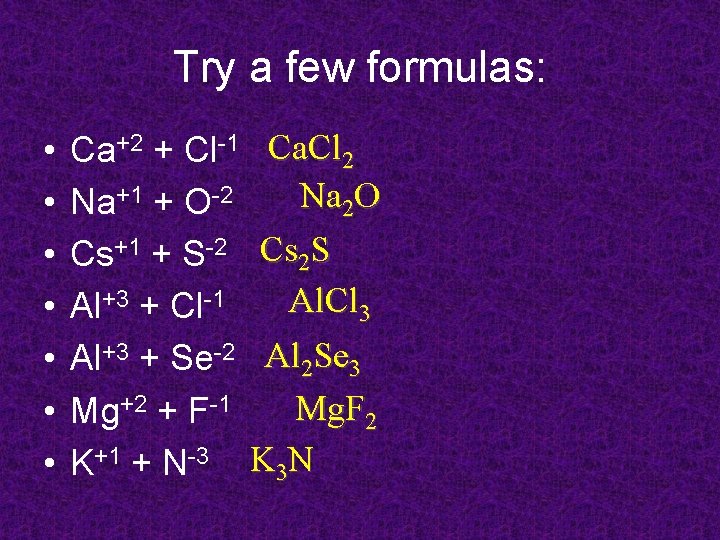
Try a few formulas: • • Ca+2 + Cl-1 Ca. Cl 2 Na 2 O Na+1 + O-2 Cs+1 + S-2 Cs 2 S Al. Cl 3 Al+3 + Cl-1 Al+3 + Se-2 Al 2 Se 3 Mg. F 2 Mg+2 + F-1 K+1 + N-3 K 3 N

Of course, it gets more difficult • • KF Potassium (K) and Fluorine (F) Zinc (Zn) and Iodine (I) Zn. I 2 Sodium (Na) and Oxygen (O) Na 2 O Magnesium (Mg) and Oxygen (O) Mg. O Aluminum (Al) and Oxygen (O) Al 2 O 3 Calcium (Ca) and Bromine (Br) Ca. Br 2 Cesium (Cs) and Iodine (I) Cs. I Silver (Ag) and Sulfur (S) Ag 2 S
- Slides: 15