Ionic Compounds with Polyatomic Ions Polyatomic Ions A






- Slides: 9

Ionic Compounds with Polyatomic Ions

Polyatomic Ions A group of atoms with an overall charge. NH 4+ ammonium OH- hydroxide NO 3 - nitrate NO 2 - nitrite CO 32 - Carbonate HCO 3 - hydrogen carbonate (bicarbonate)

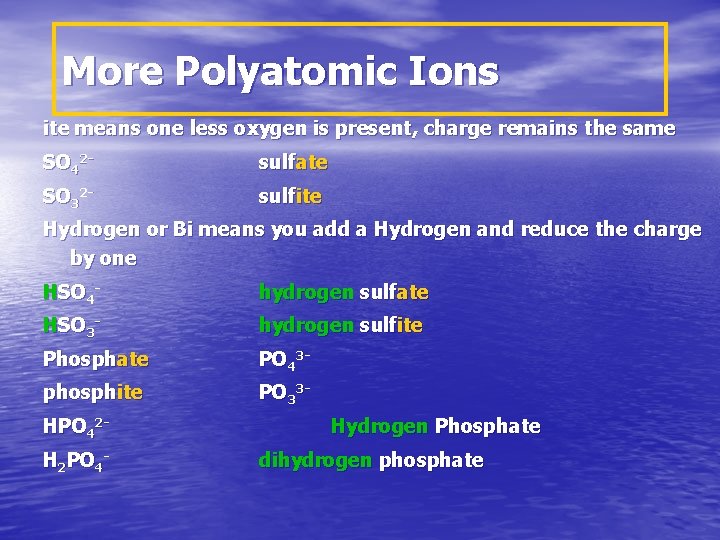
More Polyatomic Ions ite means one less oxygen is present, charge remains the same SO 42 - sulfate SO 32 - sulfite Hydrogen or Bi means you add a Hydrogen and reduce the charge by one HSO 4 - hydrogen sulfate HSO 3 - hydrogen sulfite Phosphate PO 43 - phosphite PO 33 - HPO 42 H 2 PO 4 - Hydrogen Phosphate dihydrogen phosphate

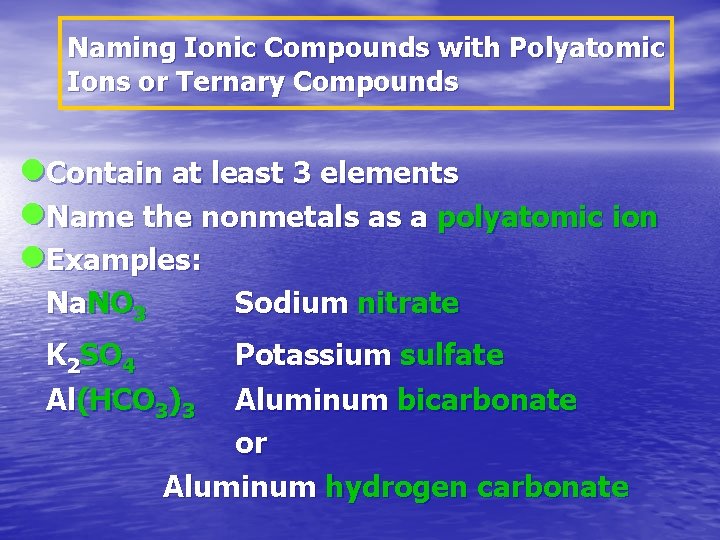
Naming Ionic Compounds with Polyatomic Ions or Ternary Compounds l. Contain at least 3 elements l. Name the nonmetals as a polyatomic ion l. Examples: Na. NO 3 Sodium nitrate K 2 SO 4 Al(HCO 3)3 Potassium sulfate Aluminum bicarbonate or Aluminum hydrogen carbonate

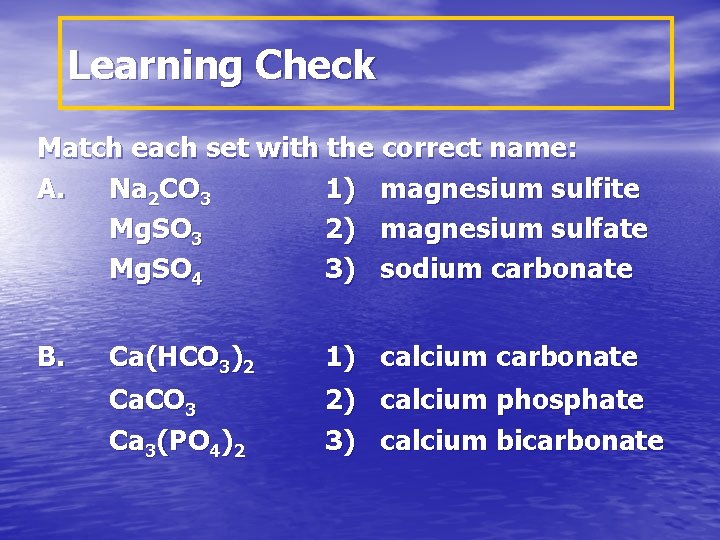
Learning Check Match each set with the correct name: A. Na 2 CO 3 1) magnesium sulfite Mg. SO 3 2) magnesium sulfate Mg. SO 4 3) sodium carbonate B. Ca(HCO 3)2 Ca. CO 3 Ca 3(PO 4)2 1) calcium carbonate 2) calcium phosphate 3) calcium bicarbonate

Solution A. B. Na 2 CO 3 Mg. SO 3 3) sodium carbonate 1) magnesium sulfite Mg. SO 4 2) magnesium sulfate Ca(HCO 3)2 3) calcium bicarbonate Ca. CO 3 Ca 3(PO 4)2 1) calcium carbonate 2) calcium phosphate

Learning Check A. aluminum nitrate 1) Al. NO 3 2) Al(NO)3 3) Al(NO 3)3 B. copper(II) nitrate 1) Cu. NO 3 2) Cu(NO 3)2 3) Cu 2(NO 3) C. Iron (III) hydroxide 1) Fe. OH 2) Fe 3 OH D. Tin(IV) hydroxide 1) Sn(OH)4 2) Sn(OH)2 3) Fe(OH)3 3) Sn 4(OH)

Solution A. aluminum nitrate 3) Al(NO 3)3 B. copper(II) nitrate 2) Cu(NO 3)2 C. Iron (III) hydroxide 3) Fe(OH)3 D. Tin(IV) hydroxide 1) Sn(OH)4

Notice • When formulas are formed, the number of Oxygens are NOT changed. • Sulfate has 4 Oxygens and is written SO 4 if there is one, two or five present. • Number of SO 4 ‘s present is shown by placing the Polyatomic in parenthesis and the amount is placed outside • 2 Sulfates would be written as (SO 4)2