Ionic Compounds Types of bonds A chemical bond
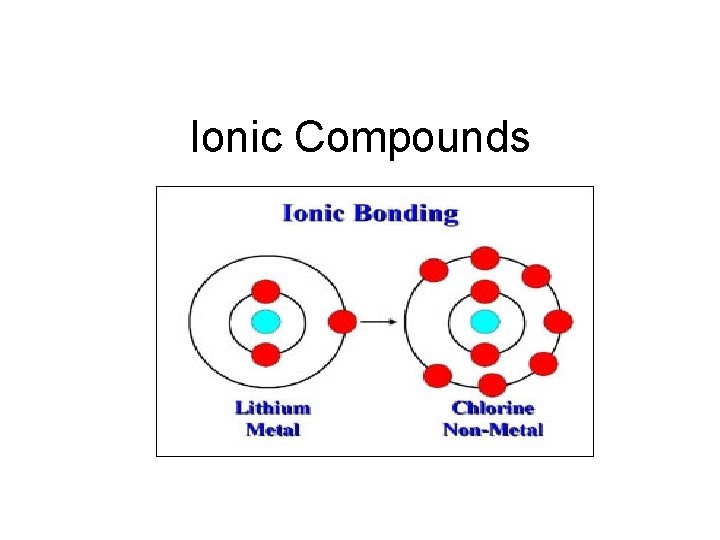
Ionic Compounds
Types of bonds • A chemical bond is the force that holds atoms together in a molecule. • Ionic bonds form when electrons are transferred between atoms. • Covalent bonds form when electrons are shared between atoms.
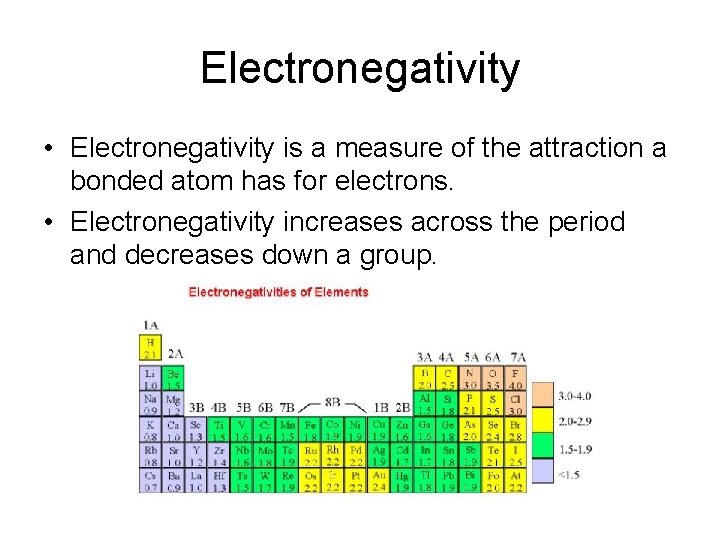
Electronegativity • Electronegativity is a measure of the attraction a bonded atom has for electrons. • Electronegativity increases across the period and decreases down a group.
Formation of Ionic Bonds • If the electronegativity difference is large (a metal with a nonmetal) an ionic bond will form. • Ionic bonds are the strongest type of bond. • Substances resulting from ionic bonds are classified as crystalline solids.

Let’s Practice • Identify the type of bond (ionic or covalent) that will form between each pair of atoms. 1) Na and Cl 2) C and O 3) N and H 4) Ca and O 5) K and N 6) Mg and F
Example
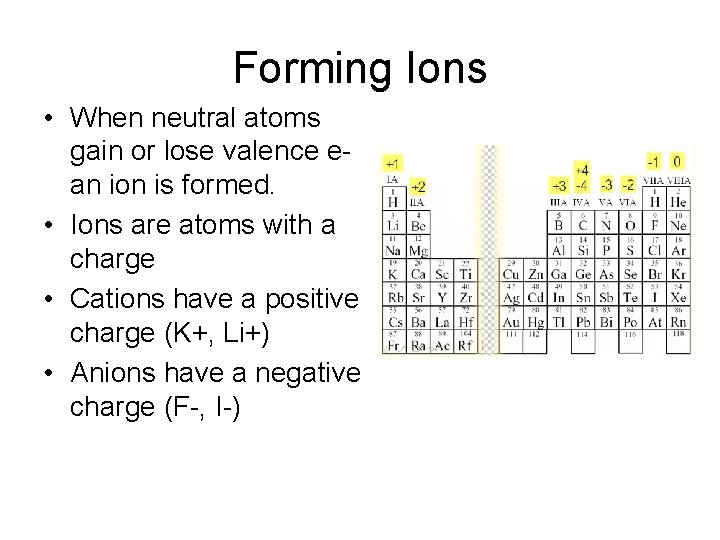
Forming Ions • When neutral atoms gain or lose valence ean ion is formed. • Ions are atoms with a charge • Cations have a positive charge (K+, Li+) • Anions have a negative charge (F-, I-)

Ions forming bonds • Cations are attracted to anions because of opposite charges. • “Opposites attract”
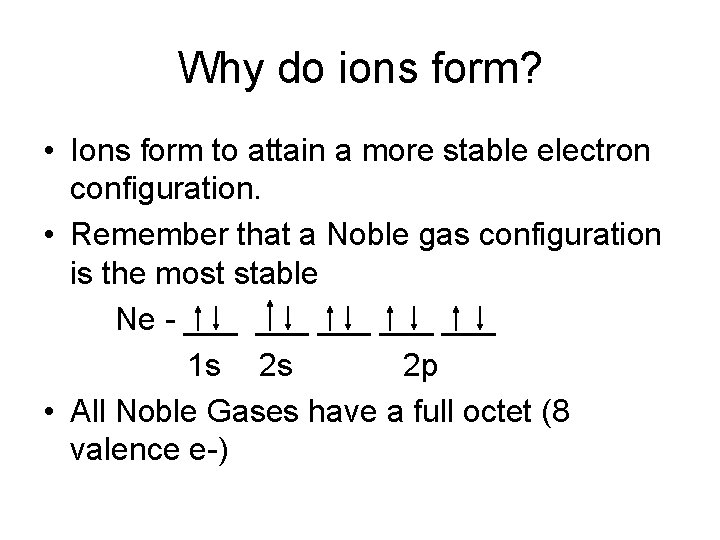
Why do ions form? • Ions form to attain a more stable electron configuration. • Remember that a Noble gas configuration is the most stable Ne - ___ ___ ___ 1 s 2 s 2 p • All Noble Gases have a full octet (8 valence e-)
Noble Gases

Why do ions form? Na __ __ __ 1 s 2 s 2 p 3 s Na+ __ __ __ 1 s 2 s 2 p 3 s Now Na+ looks like neon Ne __ __ __ 1 s 2 s 2 p • All atoms are trying to have a Noble Gas electron configuration so, they will lose or gain electrons to get there
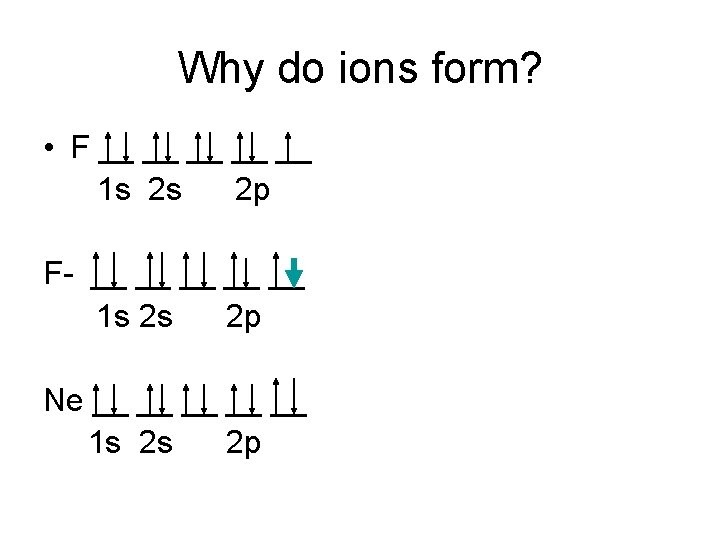
Why do ions form? • F __ __ __ 1 s 2 s 2 p F- __ __ __ 1 s 2 s 2 p Ne __ __ __ 1 s 2 s 2 p

Review Draw the dot notation for each element and the charge of its ion • N • S • Ba • Li
Ionic Bonds • Ionic bonds- the force that holds two oppositely (+ and -) charged particles together. • Ionic compounds contain ionic bonds and are neutral • Usually forms between a metal and a nonmetal
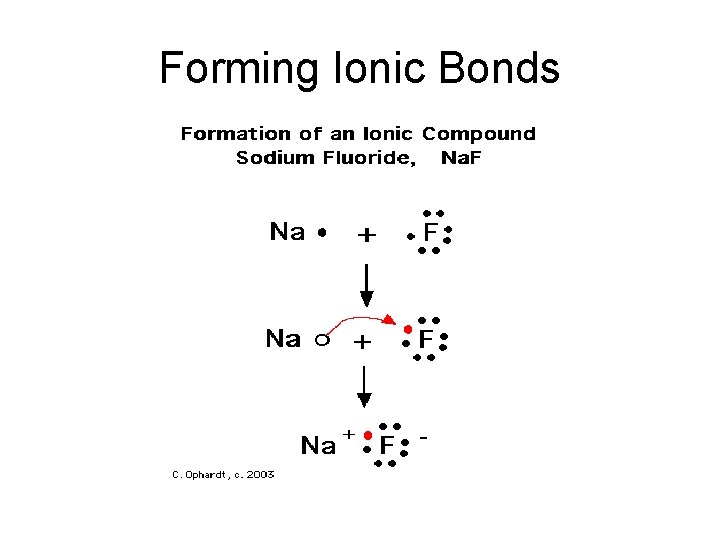
Forming Ionic Bonds
Forming Ionic Compounds Show ionic compounds are formed for the following elements. 1. Na and I 2. Mg and O 3. Al and Cl

Ionization Energy • Energy needed to remove the outermost electron. • Low ionization energy indicates an atom’s nucleus has a weaker hold on its electrons These atoms will lose electrons easily Metals = have low IE, therefore lose electrons (become cations)

Ionization Energy • High ionization energy indicates an atom’s nucleus holds on strongly to its electrons These atoms will not lose electrons Small atoms have high IE Nonmetals = have high IE, therefore gain electrons (become anions)

Ionization Energy Trend • Increases across the period – atoms get smaller as you move across a period. Increased positive nuclear charge holds tightly to electrons. • Decreases down the group – atoms get larger as you move down a group. Valence electrons are further from positive nuclear charge, so not held as tightly. Easier to remove.
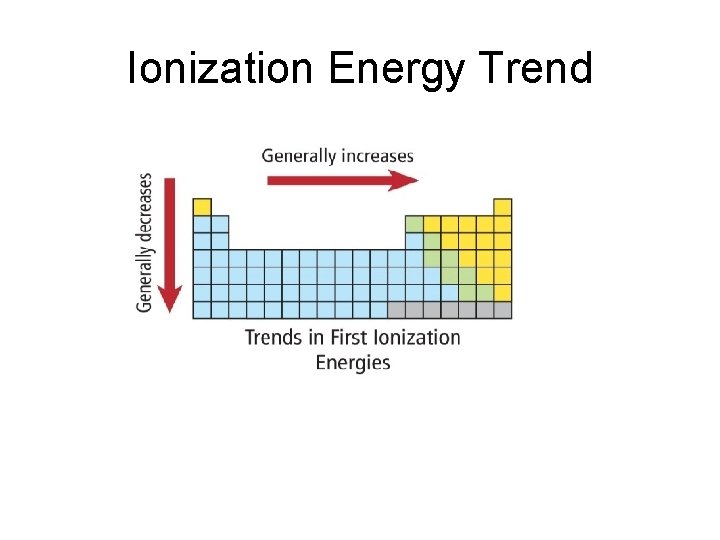
Ionization Energy Trend

Ionization Energy Trend • What do you notice about how the ionization energy trend compares to the electronegativity trend? • Which element has the highest ionization energy? – Ca or Br – N or Sb

Ionic Radius • Cations have lost electrons to become positively charged– they become smaller than their parent atom. • Anions have gained electrons to become negatively charged – they become larger than their parent atom.

Ionic Radius
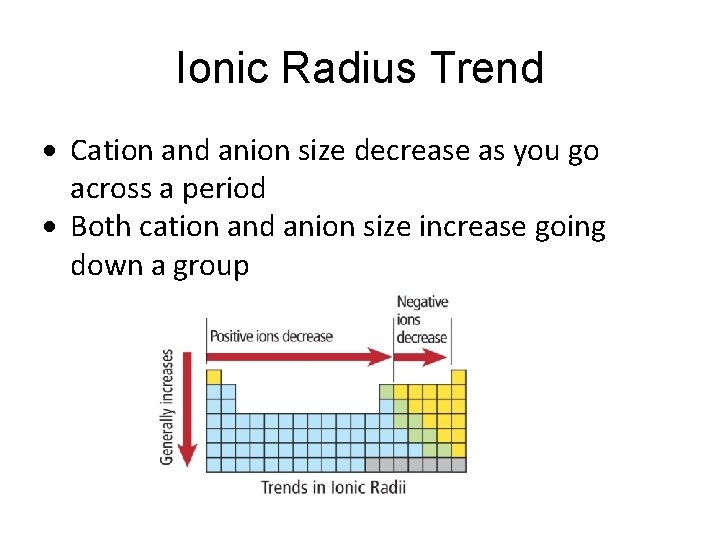
Ionic Radius Trend Cation and anion size decrease as you go across a period Both cation and anion size increase going down a group

Ionic Radius • Which ionic radius would be bigger? – Ca or Ca+2 – O or O-2
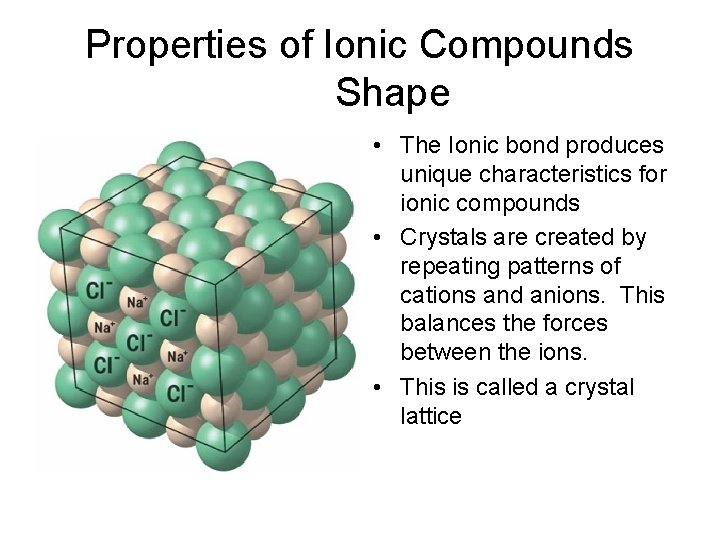
Properties of Ionic Compounds Shape • The Ionic bond produces unique characteristics for ionic compounds • Crystals are created by repeating patterns of cations and anions. This balances the forces between the ions. • This is called a crystal lattice

Properties of Ionic Compounds Physical Properties • Electrolytes (aqueous ions- Ions dissolved in water) conduct electricity • Solid ionic compounds DO NOT conduct electricity because the charges are locked in place

Properties of Ionic Compounds Physical Properties • Ionic solids are strong and hard to break apart • High Melting points • High Boiling points • Transition metals in the crystal give the ionic solid color.

Properties of Ionic Compounds Physical Properties The solid ionic crystals are hard, rigid, and brittle The strong forces between ions produce this property
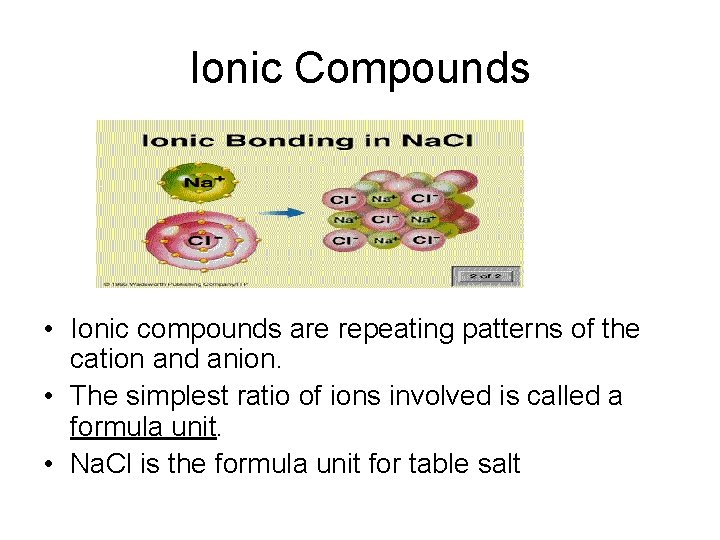
Ionic Compounds • Ionic compounds are repeating patterns of the cation and anion. • The simplest ratio of ions involved is called a formula unit. • Na. Cl is the formula unit for table salt
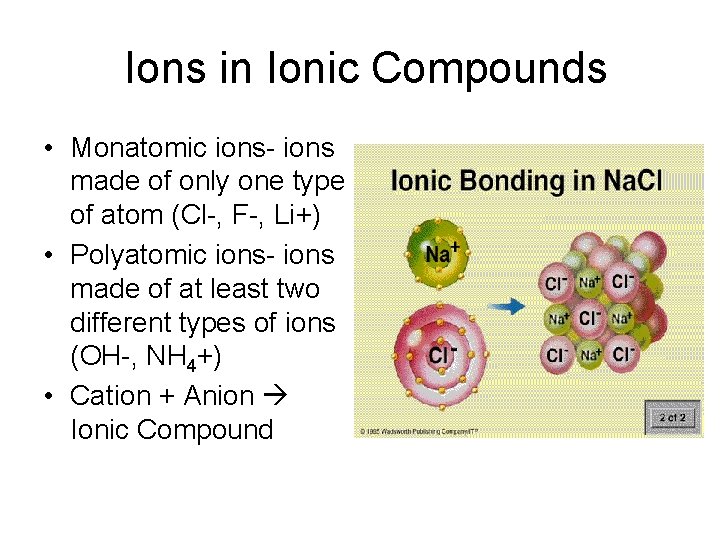
Ions in Ionic Compounds • Monatomic ions- ions made of only one type of atom (Cl-, F-, Li+) • Polyatomic ions- ions made of at least two different types of ions (OH-, NH 4+) • Cation + Anion Ionic Compound
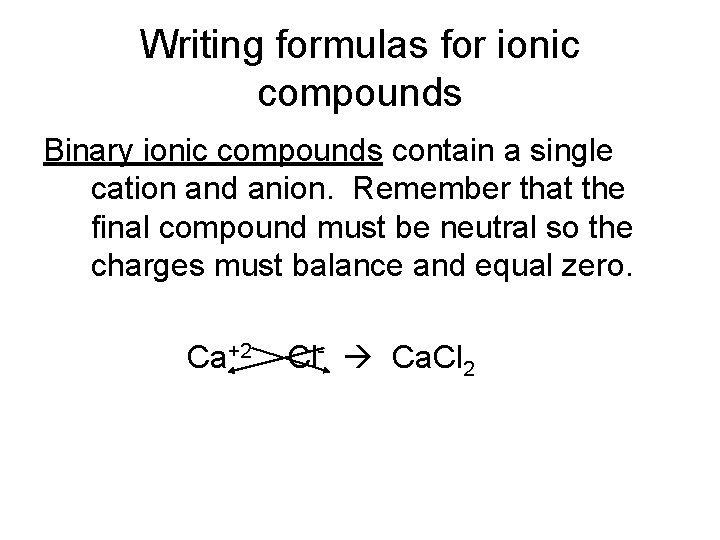
Writing formulas for ionic compounds Binary ionic compounds contain a single cation and anion. Remember that the final compound must be neutral so the charges must balance and equal zero. Ca+2 Cl- Ca. Cl 2
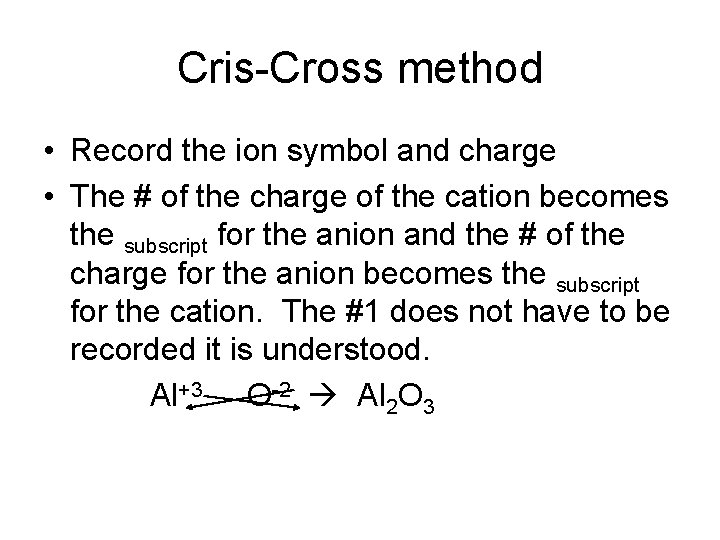
Cris-Cross method • Record the ion symbol and charge • The # of the charge of the cation becomes the subscript for the anion and the # of the charge for the anion becomes the subscript for the cation. The #1 does not have to be recorded it is understood. Al+3 O-2 Al 2 O 3
Cris-Cross method Al 2 O 3 is the formula unit for aluminum oxide. The subscripts indicate how many ions are needed of each in order to balance the charges.

Try a few • • Potassium and Iodine Magnesium and chlorine Aluminum and bromine Cesium and nitrogen

Naming Ionic Compounds Na. Br • Name the cation the anion. Cations are always written first • Monatomic cations always keep their name. If the cation is an ion that may carry multiple charges then a roman numeral is used to indicate which charge the cation is carrying. [Fe+3 (III) and Fe+2 (II)].

Naming Ionic Compounds The monatomic anion’s ending is changed to -ide. Fluorine is changed to Fluoride. Na. Br- sodium bromide Fe 3 N 2 – Iron (II) nitride

Let’s practice • • Ca. Cl 2 K 2 O Cu. Cl 2 Fe 2 O 3

What about Polyatomic ions? • Al+3 and PO 4 -3 Al. PO 4 • The same rules apply, however NEVER CHANGE A SUBSCRIPT THAT IS ALREADY THERE. • Polyatomic ions may also require () around them if there is a need for more than one to balance the charge
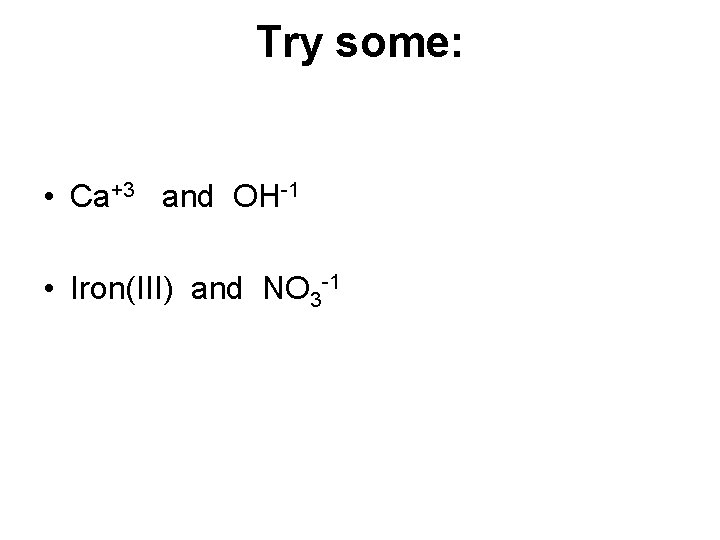
Try some: • Ca+3 and OH-1 • Iron(III) and NO 3 -1
- Slides: 40