IONIC COMPOUNDS Names and Formulas Naming compounds in





- Slides: 17

IONIC COMPOUNDS: Names and Formulas

Naming compounds in chemistry is called NOMENCLATURE

There a few general rules that apply when naming ionic compounds. 1. Most ionic compounds are also called salts. 2. Most ionic compounds exist as solids and many dissolve to form aqueous solutions. 3. An ionic compound is made up of a metal and a nonmetal; metals are located on the left side of the periodic table and nonmetals are on the right side. 4. The cation (positive ion) is written first followed by the anion (negative ion). 5. Before naming compounds, you should first memorize the individual cations and anions.

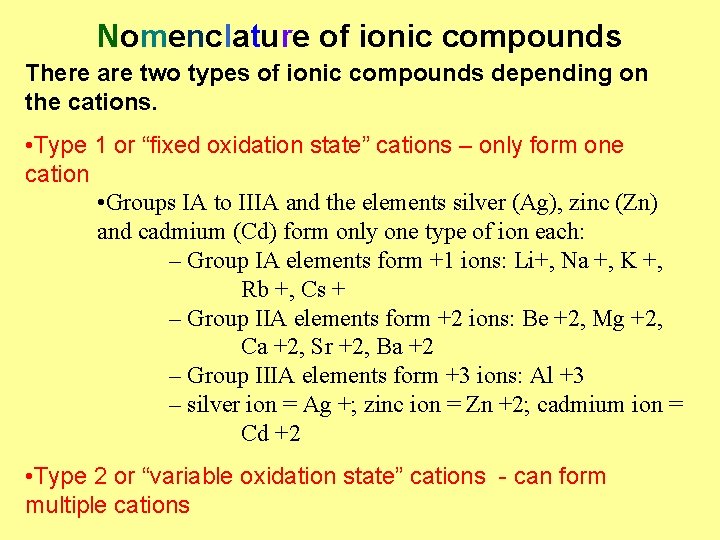
Nomenclature of ionic compounds There are two types of ionic compounds depending on the cations. • Type 1 or “fixed oxidation state” cations – only form one cation • Groups IA to IIIA and the elements silver (Ag), zinc (Zn) and cadmium (Cd) form only one type of ion each: – Group IA elements form +1 ions: Li+, Na +, K +, Rb +, Cs + – Group IIA elements form +2 ions: Be +2, Mg +2, Ca +2, Sr +2, Ba +2 – Group IIIA elements form +3 ions: Al +3 – silver ion = Ag +; zinc ion = Zn +2; cadmium ion = Cd +2 • Type 2 or “variable oxidation state” cations - can form multiple cations

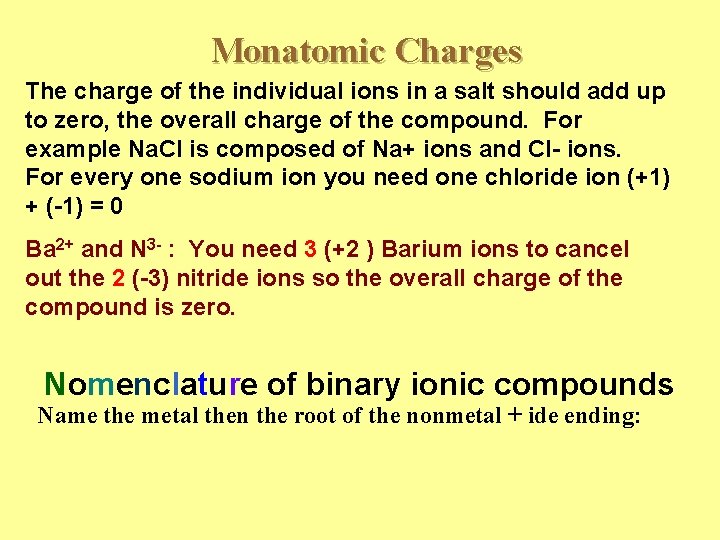
Monatomic Charges The charge of the individual ions in a salt should add up to zero, the overall charge of the compound. For example Na. Cl is composed of Na+ ions and Cl- ions. For every one sodium ion you need one chloride ion (+1) + (-1) = 0 Ba 2+ and N 3 - : You need 3 (+2 ) Barium ions to cancel out the 2 (-3) nitride ions so the overall charge of the compound is zero. Nomenclature of binary ionic compounds Name the metal then the root of the nonmetal + ide ending:

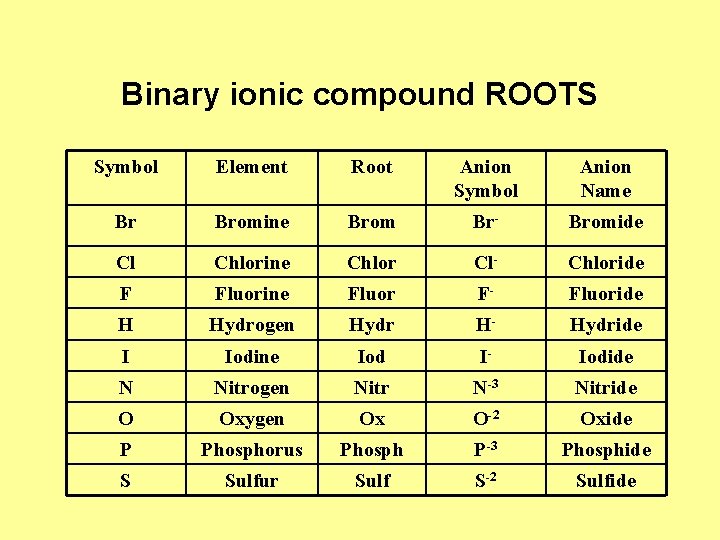
Binary ionic compound ROOTS Symbol Element Root Anion Symbol Anion Name Br Bromine Brom Br- Bromide Cl Chlorine Chlor Cl- Chloride F Fluorine Fluor F- Fluoride H Hydrogen Hydr H- Hydride I Iodine Iod I- Iodide N Nitrogen Nitr N-3 Nitride O Oxygen Ox O-2 Oxide P Phosphorus Phosph P-3 Phosphide S Sulfur Sulf S-2 Sulfide

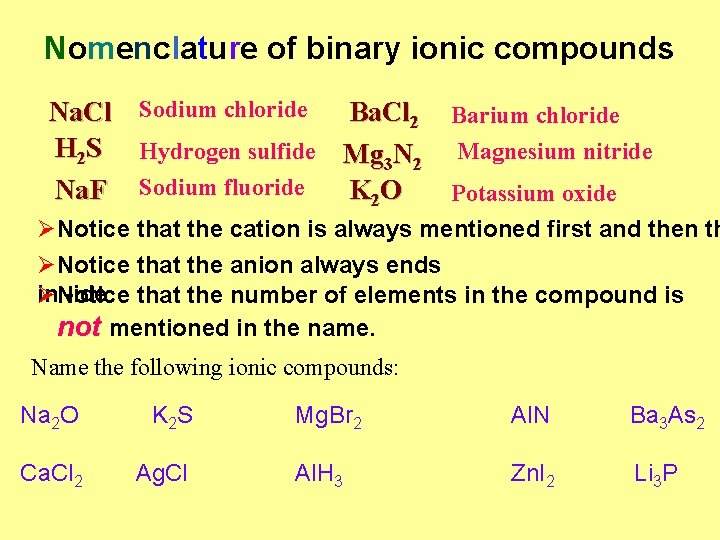
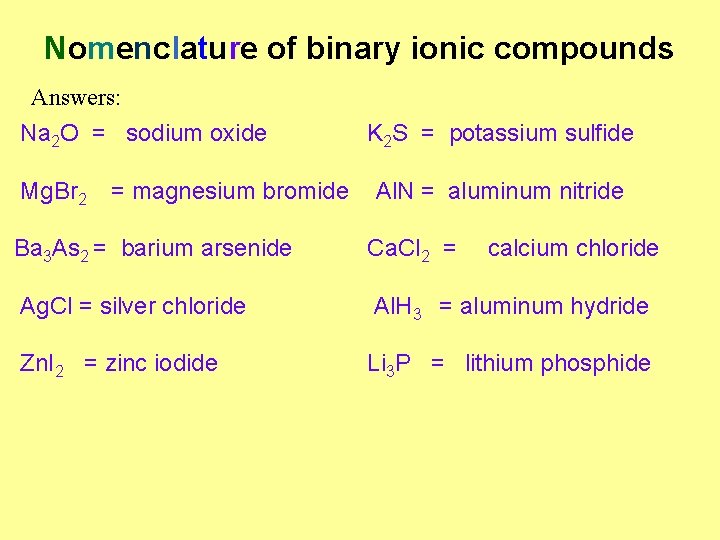
Nomenclature of binary ionic compounds Na. Cl Sodium chloride Ba. Cl 2 H 2 S Hydrogen sulfide Mg 3 N 2 Na. F Sodium fluoride K 2 O Barium chloride Magnesium nitride Potassium oxide ØNotice that the cation is always mentioned first and then th ØNotice that the anion always ends in -ide that the number of elements in the compound is ØNotice not mentioned in the name. Name the following ionic compounds: Na 2 O K 2 S Ca. Cl 2 Ag. Cl Mg. Br 2 Al. N Ba 3 As 2 Al. H 3 Zn. I 2 Li 3 P

Nomenclature of binary ionic compounds Answers: Na 2 O = sodium oxide Mg. Br 2 = magnesium bromide K 2 S = potassium sulfide Al. N = aluminum nitride Ba 3 As 2 = barium arsenide Ca. Cl 2 = calcium chloride Ag. Cl = silver chloride Al. H 3 = aluminum hydride Zn. I 2 = zinc iodide Li 3 P = lithium phosphide

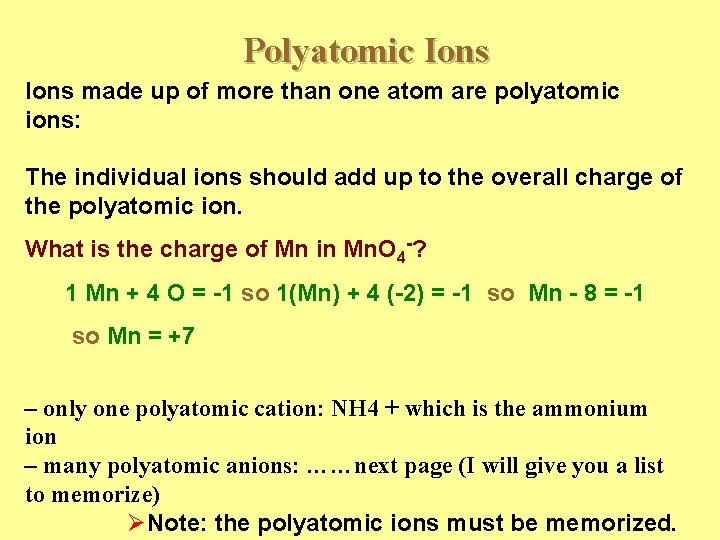
Polyatomic Ions made up of more than one atom are polyatomic ions: The individual ions should add up to the overall charge of the polyatomic ion. What is the charge of Mn in Mn. O 4 -? 1 Mn + 4 O = -1 so 1(Mn) + 4 (-2) = -1 so Mn - 8 = -1 so Mn = +7 – only one polyatomic cation: NH 4 + which is the ammonium ion – many polyatomic anions: ……next page (I will give you a list to memorize) ØNote: the polyatomic ions must be memorized.

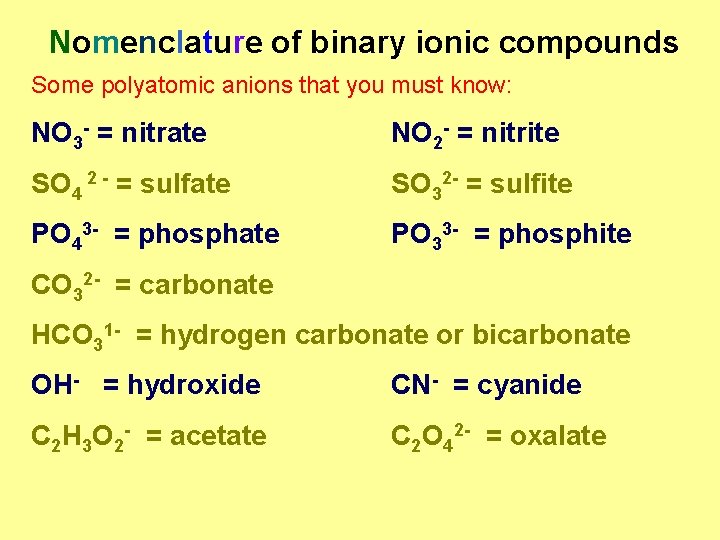
Nomenclature of binary ionic compounds Some polyatomic anions that you must know: NO 3 - = nitrate NO 2 - = nitrite SO 4 2 - = sulfate SO 32 - = sulfite PO 43 - = phosphate PO 33 - = phosphite CO 32 - = carbonate HCO 31 - = hydrogen carbonate or bicarbonate OH- = hydroxide CN- = cyanide C 2 H 3 O 2 - = acetate C 2 O 42 - = oxalate

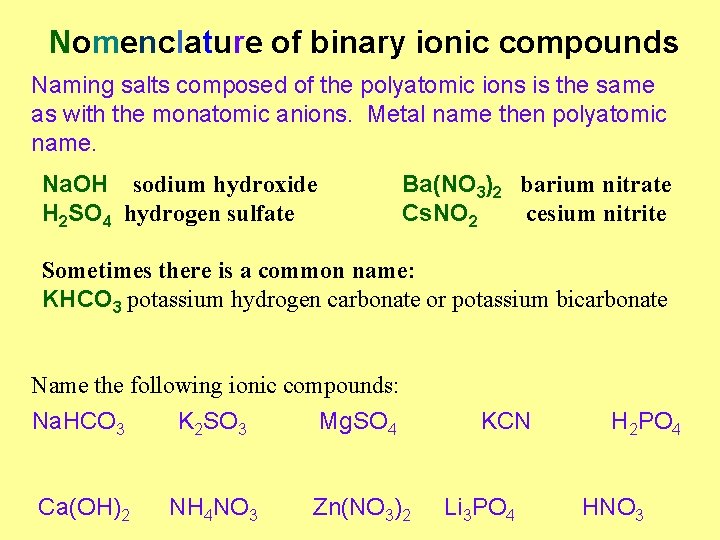
Nomenclature of binary ionic compounds Naming salts composed of the polyatomic ions is the same as with the monatomic anions. Metal name then polyatomic name. Na. OH sodium hydroxide H 2 SO 4 hydrogen sulfate Ba(NO 3)2 barium nitrate Cs. NO 2 cesium nitrite Sometimes there is a common name: KHCO 3 potassium hydrogen carbonate or potassium bicarbonate Name the following ionic compounds: Na. HCO 3 K 2 SO 3 Mg. SO 4 Ca(OH)2 NH 4 NO 3 Zn(NO 3)2 KCN Li 3 PO 4 H 2 PO 4 HNO 3

Nomenclature of binary ionic compounds Answers: Na. HCO 3 = sodium hydrogen carbonate or sodium bicarbonate K 2 SO 3 = potassium sulfite Mg. SO 4 = magnesium sulfate KCN = potassium cyanide H 2 PO 4 = hydrogen phosphate Ca(OH)2 = calcium hydroxide NH 4 NO 3 = ammonium nitrate Zn(NO 3)2 = zinc nitrate Li 3 PO 4 = lithium phosphate HNO 3 = hydrogen nitrate

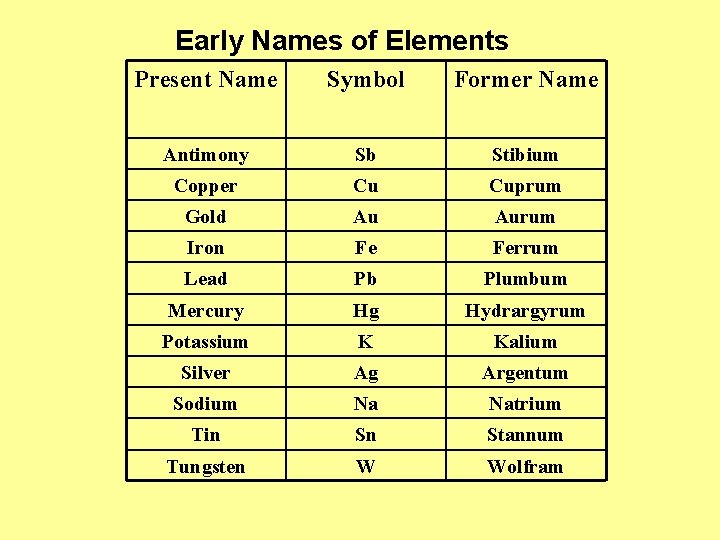
Early Names of Elements Present Name Symbol Former Name Antimony Sb Stibium Copper Cu Cuprum Gold Au Aurum Iron Fe Ferrum Lead Pb Plumbum Mercury Hg Hydrargyrum Potassium K Kalium Silver Ag Argentum Sodium Na Natrium Tin Sn Stannum Tungsten W Wolfram

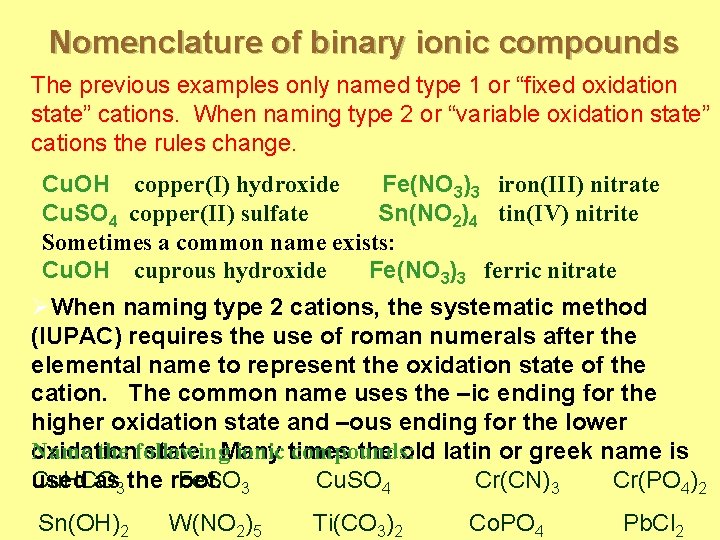
Nomenclature of binary ionic compounds The previous examples only named type 1 or “fixed oxidation state” cations. When naming type 2 or “variable oxidation state” cations the rules change. Cu. OH copper(I) hydroxide Fe(NO 3)3 iron(III) nitrate Cu. SO 4 copper(II) sulfate Sn(NO 2)4 tin(IV) nitrite Sometimes a common name exists: Cu. OH cuprous hydroxide Fe(NO 3)3 ferric nitrate ØWhen naming type 2 cations, the systematic method (IUPAC) requires the use of roman numerals after the elemental name to represent the oxidation state of the cation. The common name uses the –ic ending for the higher oxidation state and –ous ending for the lower Name the following ionic times compounds: oxidation state. Many the old latin or greek name is used as 3 the root. Cu. HCO Fe. SO 3 Cu. SO 4 Cr(CN)3 Cr(PO 4)2 Sn(OH)2 W(NO 2)5 Ti(CO 3)2 Co. PO 4 Pb. Cl 2

Nomenclature of binary ionic compounds Answers: Cu. HCO 3 = copper(I) hydrogen carbonate or cuprous bicarbonate Fe. SO 3 = iron(II) sulfite or ferrous sulfite Cu. SO 4 = copper(II) sulfate or cupric sulfate Cr(CN)3 = chromium(III) cyanide Cr(PO 4)2 = chromium (VI) phosphate Sn(OH)2 = tin(II) hydroxide or stannous hydroxide W(NO 2)5 = tungsten(V) nitrite Ti(CO 3)2 = titanium(IV) carbonate Co. PO 4 Pb. Cl 2 = cobalt(III) phosphate or cobaltic phosphate = lead(II) chloride or plumbous chloride

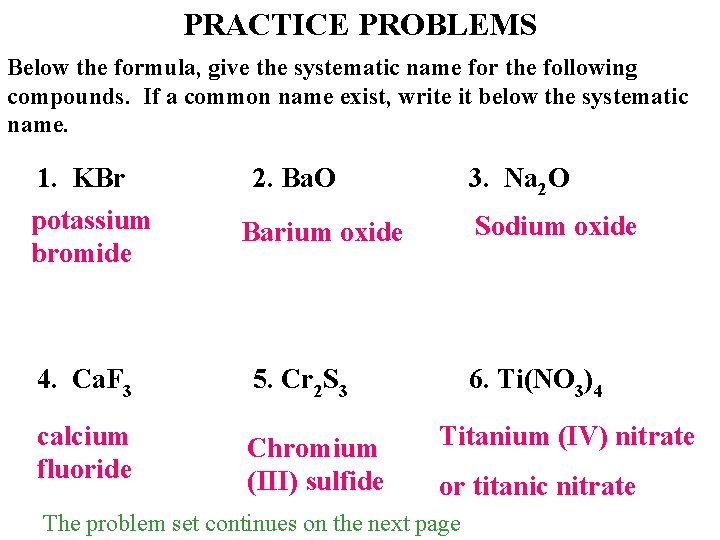
PRACTICE PROBLEMS Below the formula, give the systematic name for the following compounds. If a common name exist, write it below the systematic name. 1. KBr potassium bromide 2. Ba. O 3. Na 2 O Sodium oxide Barium oxide 4. Ca. F 3 5. Cr 2 S 3 calcium fluoride Chromium (III) sulfide 6. Ti(NO 3)4 Titanium (IV) nitrate or titanic nitrate The problem set continues on the next page.

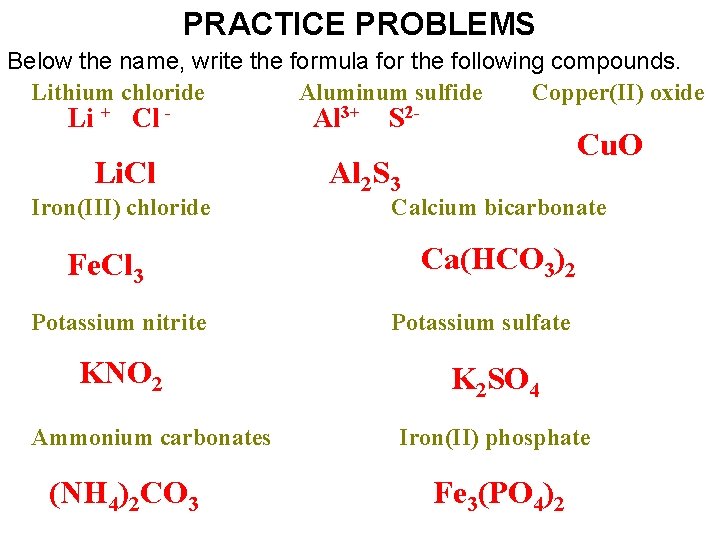
PRACTICE PROBLEMS Below the name, write the formula for the following compounds. Lithium chloride b) Aluminum sulfide e) Copper(II) oxide Li Al 3+ S 2 f) + Cl - Li. Cl Iron(III) chloride i) Fe. Cl 3 Potassium nitrite KNO 2 Ammonium carbonates s (NH 4)2 CO 3 Cu. O Al 2 S 3 Calcium bicarbonate l Ca(HCO 3)2 Potassium sulfate K 2 SO 4 )Iron(II) phosphate w) Fe 3(PO 4)2