Ionic compounds names and formulas Ionic Bonding electrical

Ionic compounds names and formulas

�Ionic Bonding – electrical attraction between large numbers of cations and anions (metals & nonmetals); forms solid crystals

Writing formulas & names � Cation anion formula name
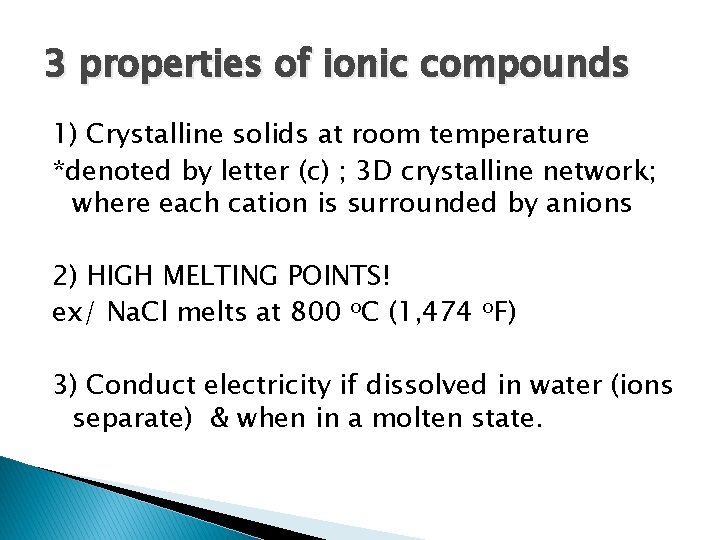
3 properties of ionic compounds 1) Crystalline solids at room temperature *denoted by letter (c) ; 3 D crystalline network; where each cation is surrounded by anions 2) HIGH MELTING POINTS! ex/ Na. Cl melts at 800 o. C (1, 474 o. F) 3) Conduct electricity if dissolved in water (ions separate) & when in a molten state.

Get a clicker for review
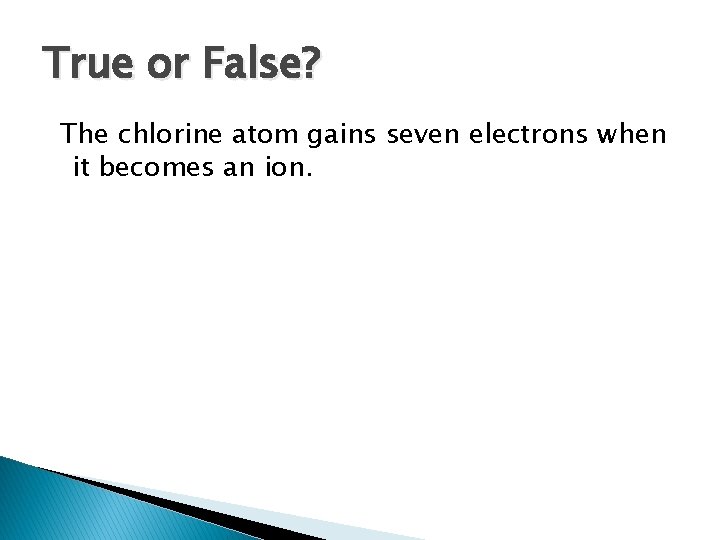
True or False? The chlorine atom gains seven electrons when it becomes an ion.

What is the formula for sodium chloride? A) B) C) D) Na. Cl Cl. Na Na 7 Cl 1 SCl
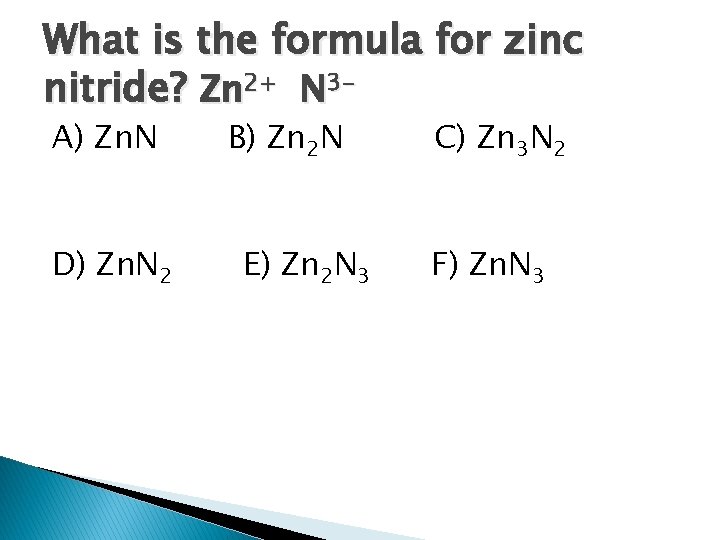
What is the formula for zinc nitride? Zn 2+ N 3 A) Zn. N D) Zn. N 2 B) Zn 2 N E) Zn 2 N 3 C) Zn 3 N 2 F) Zn. N 3

What is the name given to the electrons in the highest occupied energy level? A) B) C) D) Anions Cations Valence electrons Orbital electrons

What is the typical charge of group 2? A) B) C) D) Negative two Positive two IDK Positive one

How many valence electrons does oxygen have? A) B) C) D) None One Six eight

True or False A cation is positively charged because it will gain electrons to have a stable electron configuration like a noble gas.

True or False The name of Pb. O is lead(II) oxide

Yes or No �The charge of an anion is negative.

Please make your selection �When an aluminum atom loses its valence electrons, what is the charge on the resulting ion? �a. 2+ �b. 3+ �c. 2– �d. 1+
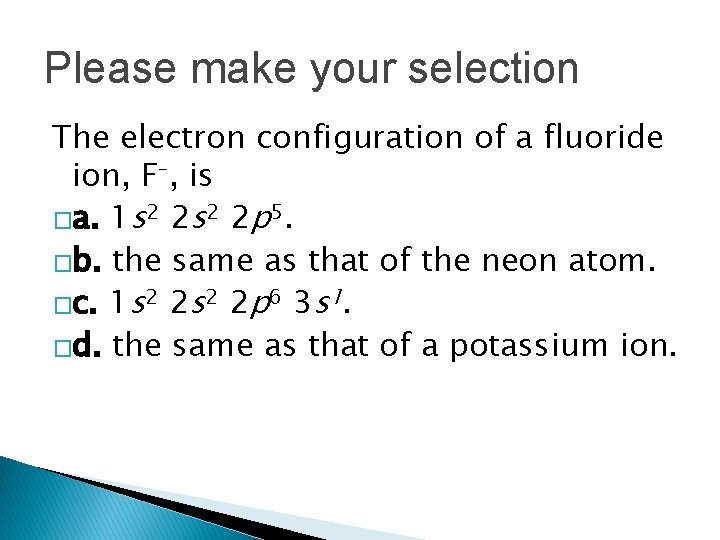
Please make your selection The electron configuration of a fluoride ion, F–, is �a. 1 s 2 2 p 5. �b. the same as that of the neon atom. �c. 1 s 2 2 p 6 3 s 1. �d. the same as that of a potassium ion.

What is the formula for iron(II) oxide? A) B) C) D) Fe 2 O 3 Fe 2 O 2 Fe. O Fe 3 O 2

Please make your selection An ionic compound is a. electrically neutral. b. composed of anions and cations. c. held together by ionic bonds d. all of the above

Please make your selection Which element when combined with chlorine would most likely form an ionic compound? a. lithium b. phosphorus c. oxygen d. bromine
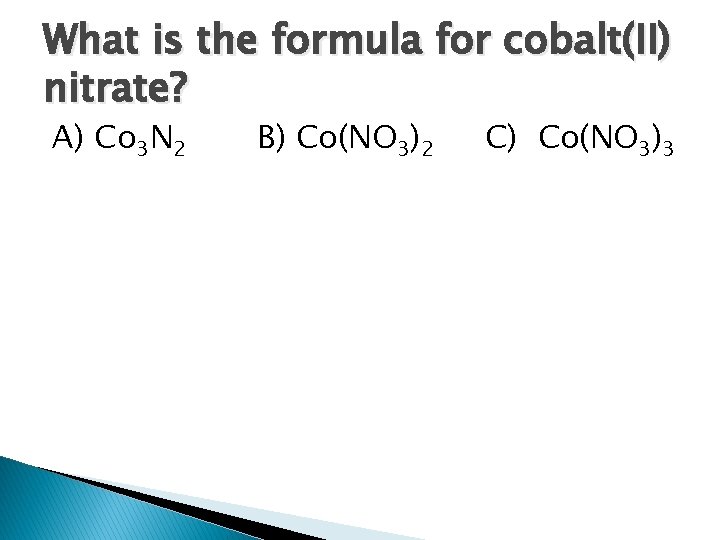
What is the formula for cobalt(II) nitrate? A) Co 3 N 2 B) Co(NO 3)2 C) Co(NO 3)3
Metallic Bonds Metallic bonding - between metal atoms; valence electrons are shared equally in a “sea of electrons” *This explains the properties of metals! � The valence electrons are mobile and can drift freely from one part of the metal to another. � Electrons are delocalized & gives metals Multidirectional strength (stretching & sheets)
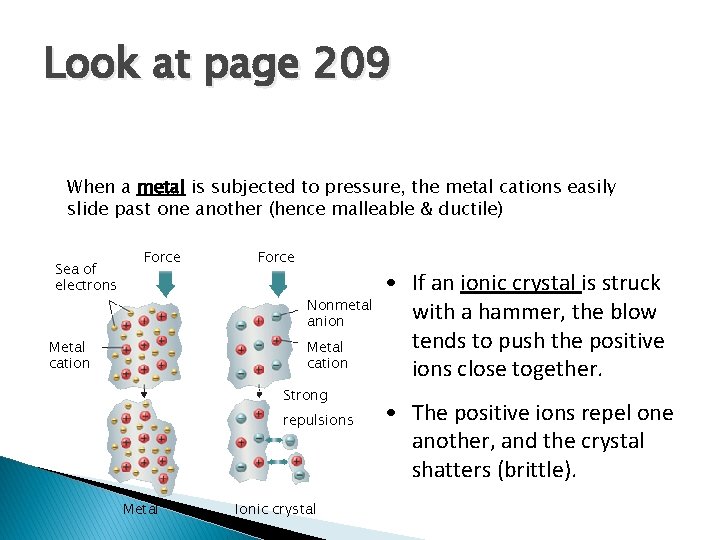
Look at page 209 When a metal is subjected to pressure, the metal cations easily slide past one another (hence malleable & ductile) Sea of electrons Force Nonmetal anion Metal cation Strong repulsions Metal Ionic crystal • If an ionic crystal is struck with a hammer, the blow tends to push the positive ions close together. • The positive ions repel one another, and the crystal shatters (brittle).
Which of the following models can describe the valence electrons of metals? A. A body-centered cube B. Octets of electrons C. A rigid array of electrons D. A sea of electrons
- Slides: 23