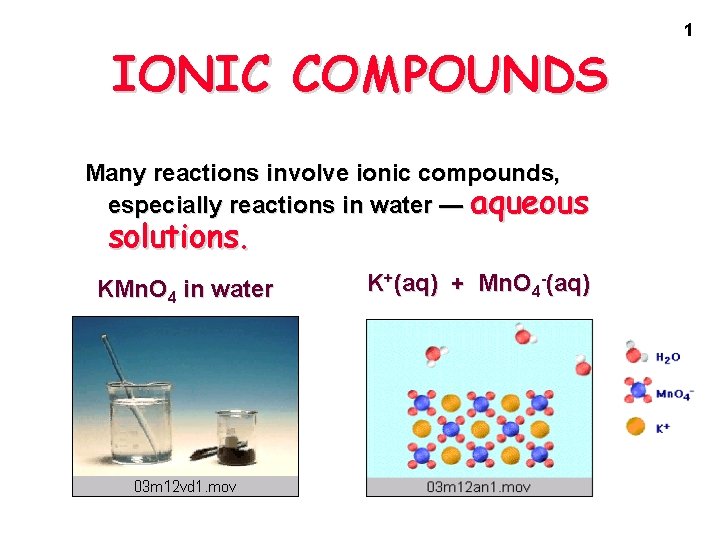
IONIC COMPOUNDS Many reactions involve ionic compounds especially







![The p. H Scale 45 p. H = log (1/ [H+]) = - log The p. H Scale 45 p. H = log (1/ [H+]) = - log](https://slidetodoc.com/presentation_image_h2/71242150eb11e63bd8f6c35e59871d8d/image-45.jpg)
![[H+] and p. H If the [H+] of soda is 1. 6 x 10 [H+] and p. H If the [H+] of soda is 1. 6 x 10](https://slidetodoc.com/presentation_image_h2/71242150eb11e63bd8f6c35e59871d8d/image-46.jpg)
![p. H and [H+] If the p. H of Coke is 3. 12, it p. H and [H+] If the p. H of Coke is 3. 12, it](https://slidetodoc.com/presentation_image_h2/71242150eb11e63bd8f6c35e59871d8d/image-47.jpg)





- Slides: 77

IONIC COMPOUNDS Many reactions involve ionic compounds, especially reactions in water — aqueous solutions. KMn. O 4 in water K+(aq) + Mn. O 4 -(aq) 1

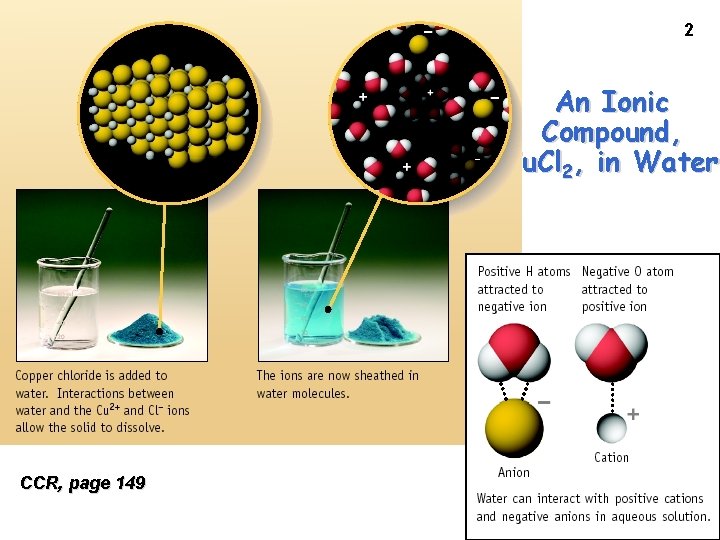
2 An Ionic Compound, Cu. Cl 2, in Water CCR, page 149

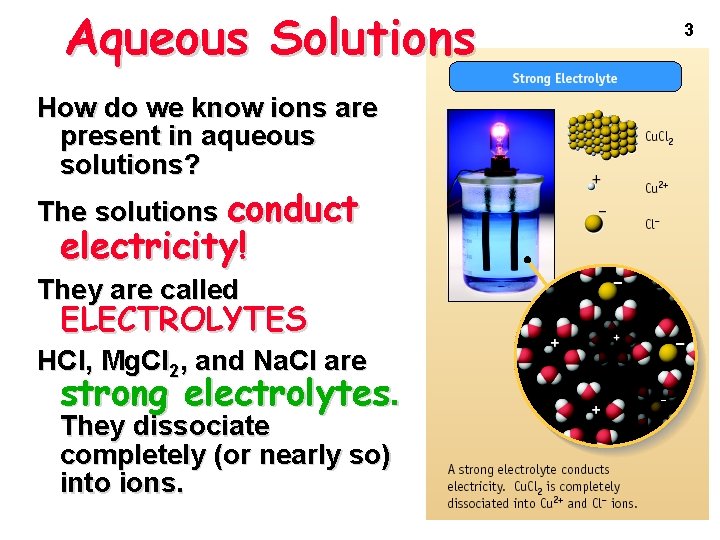
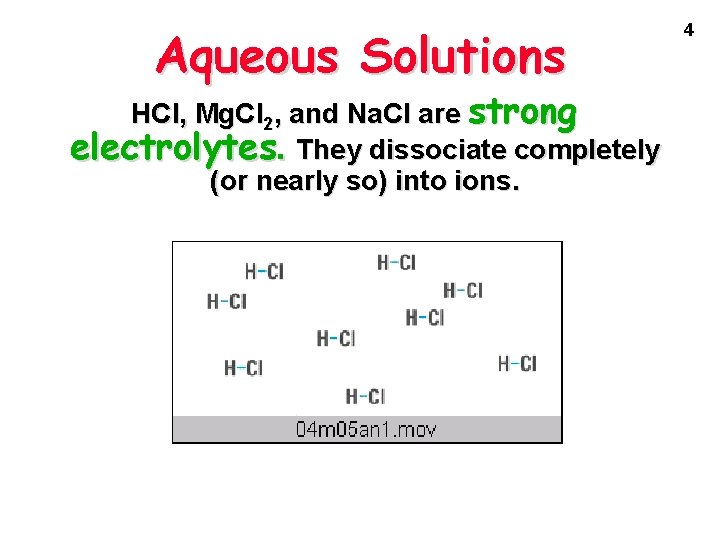
Aqueous Solutions How do we know ions are present in aqueous solutions? The solutions conduct electricity! They are called ELECTROLYTES HCl, Mg. Cl 2, and Na. Cl are strong electrolytes. They dissociate completely (or nearly so) into ions. 3

Aqueous Solutions HCl, Mg. Cl 2, and Na. Cl are strong electrolytes. They dissociate completely (or nearly so) into ions. 4

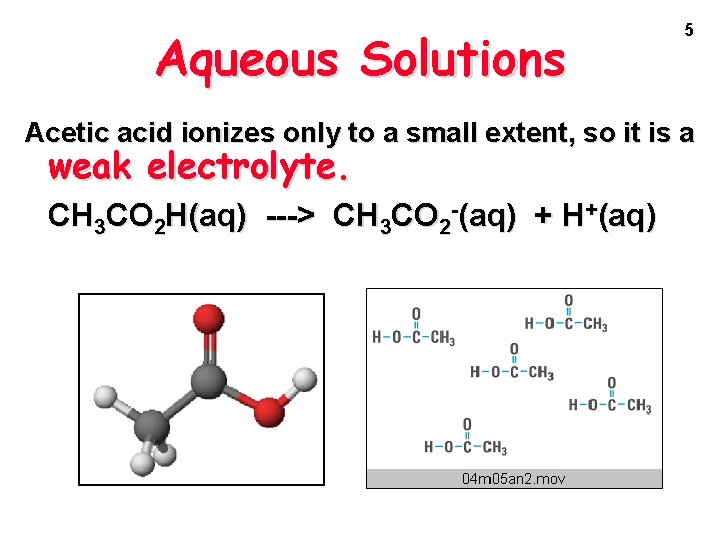
Aqueous Solutions 5 Acetic acid ionizes only to a small extent, so it is a weak electrolyte. CH 3 CO 2 H(aq) ---> CH 3 CO 2 -(aq) + H+(aq)

Aqueous Solutions Acetic acid ionizes only to a small extent, so it is a weak electrolyte. CH 3 CO 2 H(aq) ---> CH 3 CO 2 -(aq) + H+(aq) 6

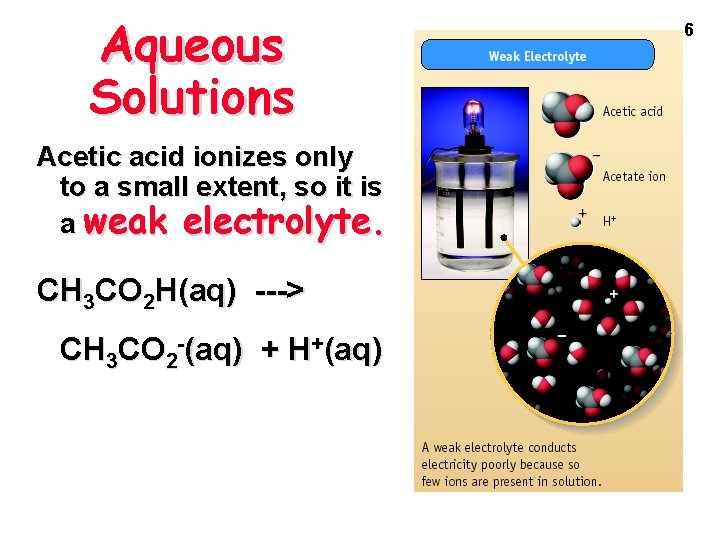
Aqueous Solutions Some compounds dissolve in water but do not conduct electricity. They are called nonelectrolytes. Examples include: sugar ethanol ethylene glycol 7

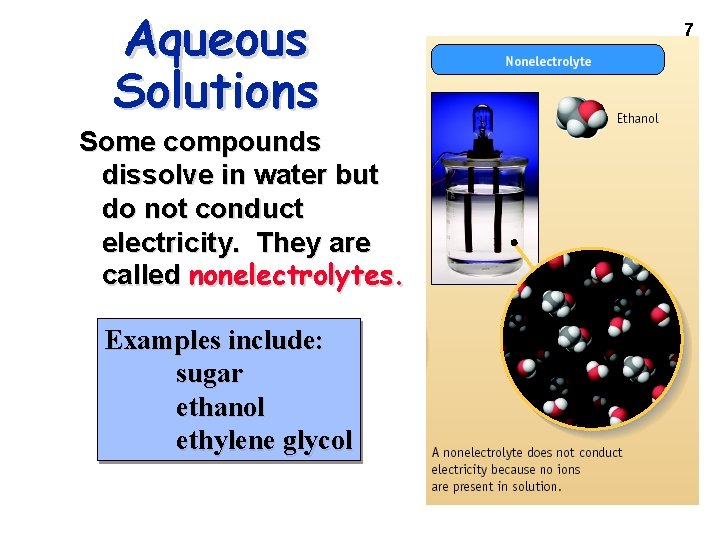
Water Solubility of Ionic Compounds 8 If one ion from the “Soluble Compd. ” list is present in a compound, the compound is water soluble. Screenof 5. 4 & Figure 5. 1 Guidelines to predict the solubility ionic compounds

9 Water Solubility of Ionic Compounds Common minerals are often formed with anions that lead to insolubility: sulfide fluoride carbonate oxide Iron pyrite, a sulfide Azurite, a copper carbonate Orpiment, arsenic sulfide

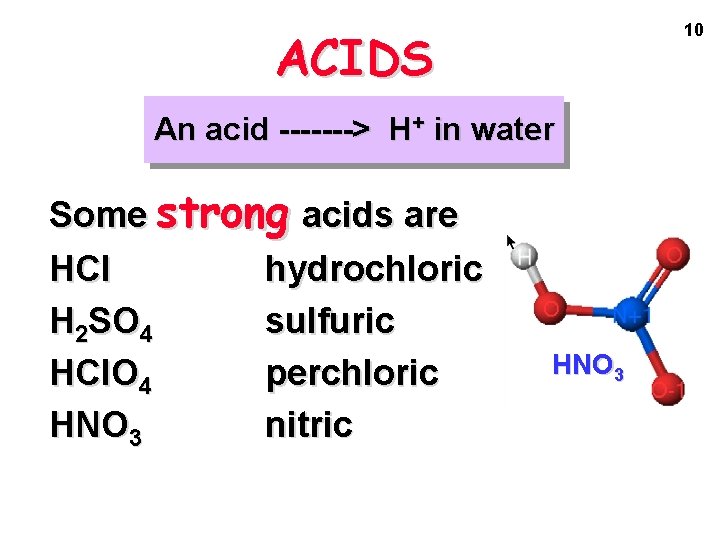
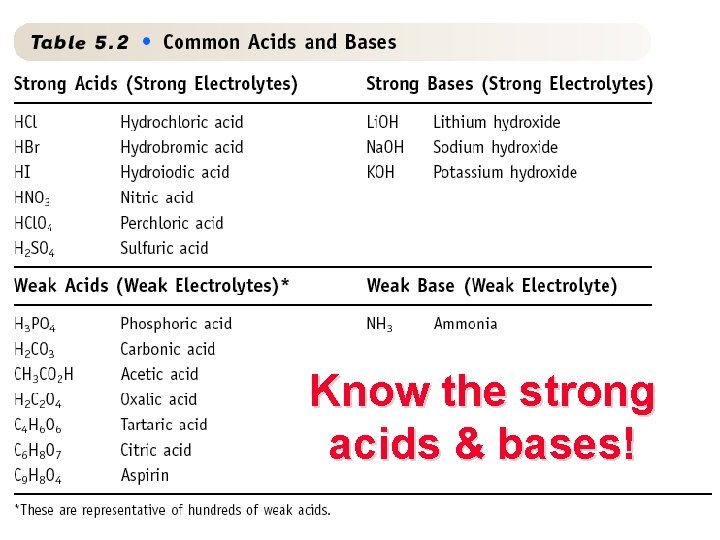
10 ACIDS An acid -------> H+ in water Some strong acids are HCl hydrochloric H 2 SO 4 sulfuric HCl. O 4 perchloric HNO 3 nitric HNO 3

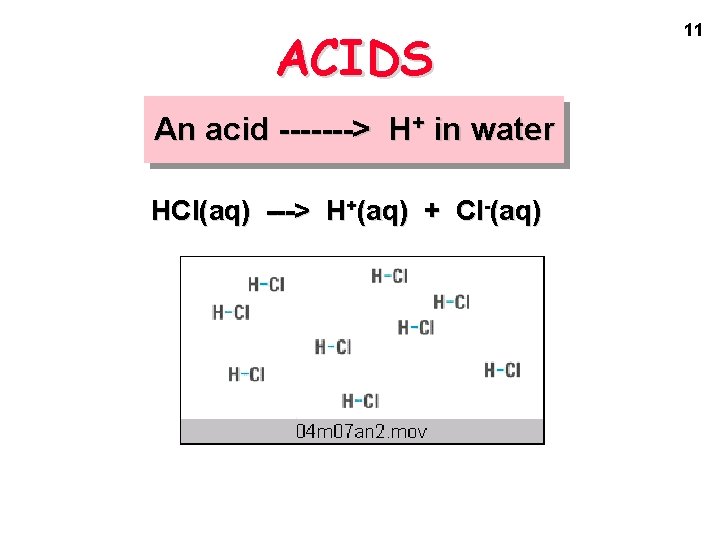
ACIDS An acid -------> H+ in water HCl(aq) ---> H+(aq) + Cl-(aq) 11

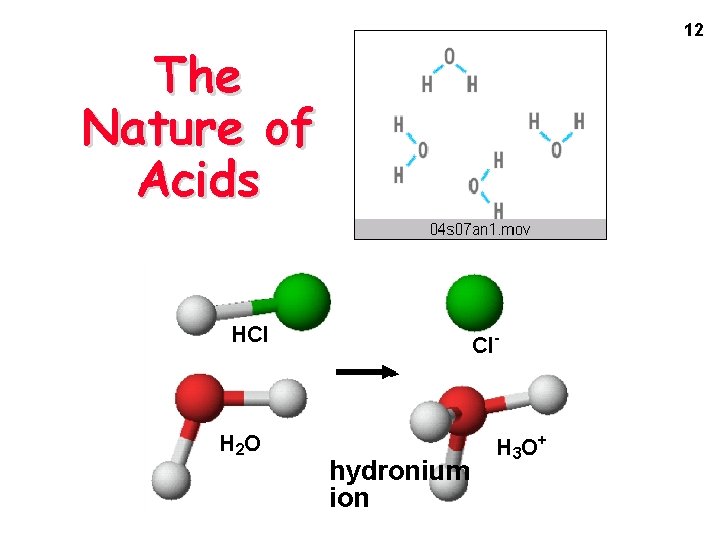
12 The Nature of Acids HCl H 2 O Cl- hydronium ion H 3 O+

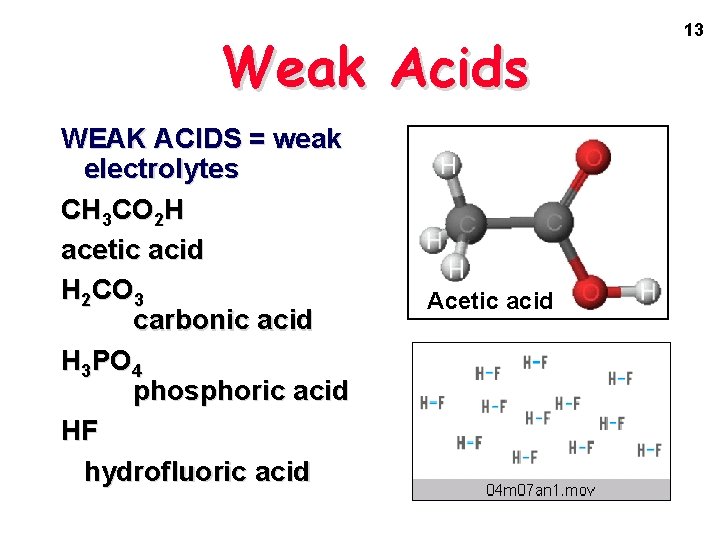
Weak Acids WEAK ACIDS = weak electrolytes CH 3 CO 2 H acetic acid H 2 CO 3 carbonic acid H 3 PO 4 phosphoric acid HF hydrofluoric acid Acetic acid 13

ACIDS Nonmetal oxides can be acids CO 2(aq) + H 2 O(liq) ---> H 2 CO 3(aq) SO 3(aq) + H 2 O(liq) ---> H 2 SO 4(aq) and can come from burning coal and oil. 14

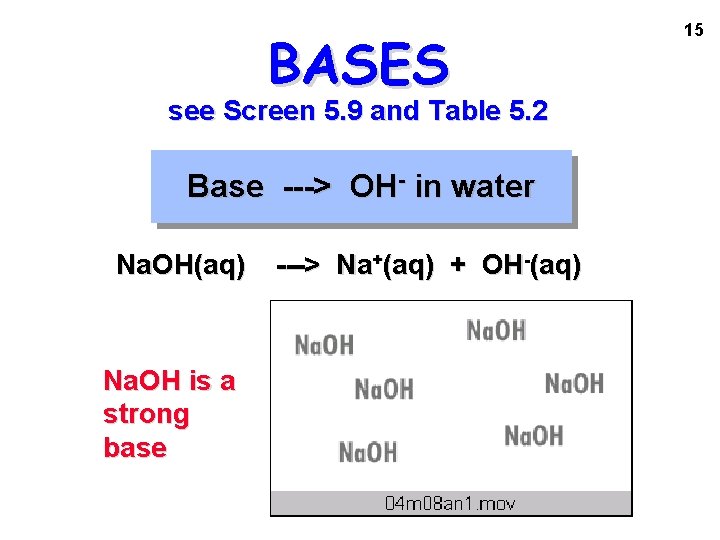
BASES see Screen 5. 9 and Table 5. 2 Base ---> OH- in water Na. OH(aq) Na. OH is a strong base ---> Na+(aq) + OH-(aq) 15

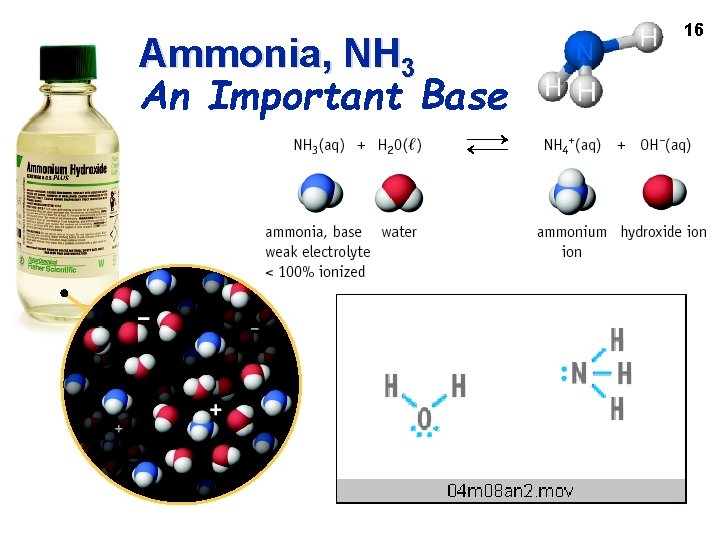
Ammonia, NH 3 An Important Base 16

BASES Metal oxides are bases Ca. O(s) + H 2 O(liq) --> Ca(OH)2(aq) Ca. O in water. Indicator shows solution is basic. 17

18 Know the strong acids & bases!

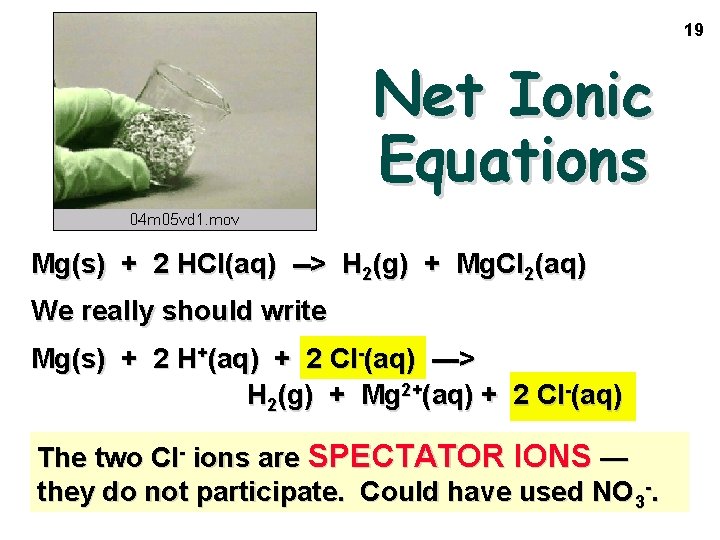
19 Net Ionic Equations Mg(s) + 2 HCl(aq) --> H 2(g) + Mg. Cl 2(aq) We really should write Mg(s) + 2 H+(aq) + 2 Cl-(aq) ---> H 2(g) + Mg 2+(aq) + 2 Cl-(aq) The two Cl- ions are SPECTATOR IONS — they do not participate. Could have used NO 3 -.

Net Ionic Equations Mg(s) + 2 HCl(aq) --> H 2(g) + Mg. Cl 2(aq) Mg(s) + 2 H+(aq) + 2 Cl-(aq) ---> H 2(g) + Mg 2+(aq) + 2 Cl-(aq) We leave the spectator ions out — Mg(s) + 2 H+(aq) ---> H 2(g) + Mg 2+(aq) to give the NET IONIC EQUATION 20

Chemical Reactions in Water 21 Sections 5. 2 & 5. 4 -5. 6—CD-ROM Ch. 5 We will look at EXCHANGE REACTIONS The anions exchange places between cations. Pb(NO 3) 2(aq) + 2 KI(aq) ----> Pb. I 2(s) + 2 KNO 3 (aq)

Precipitation Reactions The “driving force” is the formation of an insoluble compound — a precipitate. Pb(NO 3)2(aq) + 2 KI(aq) -----> 2 KNO 3(aq) + Pb. I 2(s) Net ionic equation Pb 2+(aq) + 2 I-(aq) ---> Pb. I 2(s) 22

Acid-Base Reactions • The “driving force” is the formation of water. Na. OH(aq) + HCl(aq) ---> Na. Cl(aq) + H 2 O(liq) • Net ionic equation OH-(aq) + H+(aq) ---> H 2 O(liq) • This applies to ALL reactions of STRONG acids and bases. 23

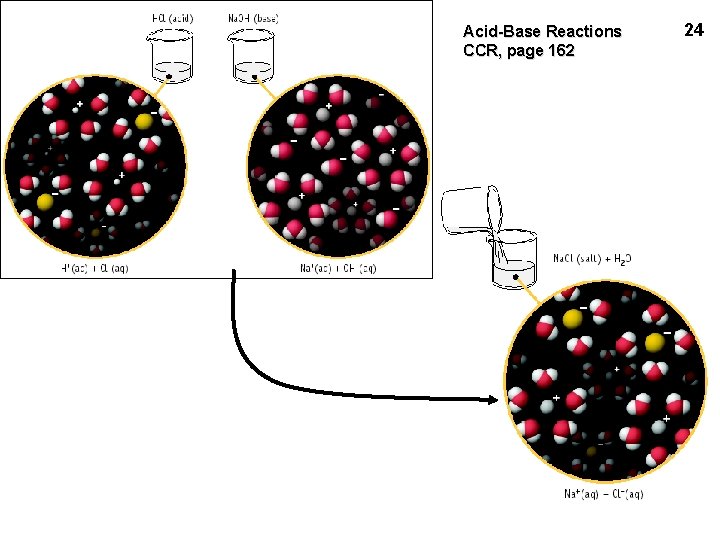
Acid-Base Reactions CCR, page 162 24

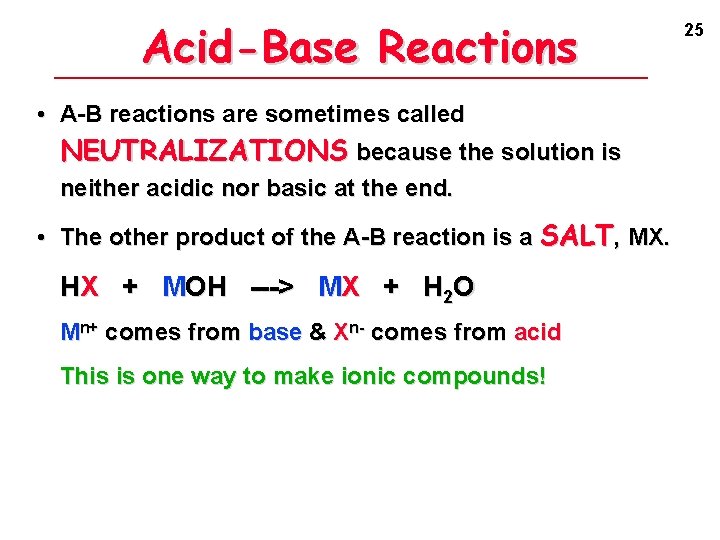
Acid-Base Reactions • A-B reactions are sometimes called NEUTRALIZATIONS because the solution is neither acidic nor basic at the end. • The other product of the A-B reaction is a SALT, MX. HX + MOH ---> MX + H 2 O Mn+ comes from base & Xn- comes from acid This is one way to make ionic compounds! 25

Gas-Forming Reactions This is primarily the chemistry of metal carbonates. CO 2 and water ---> H 2 CO 3(aq) + Ca 2+ ---> 2 H+(aq) + Ca. CO 3(s) (limestone) Adding acid reverses this reaction. MCO 3 + acid ---> CO 2 + salt 26

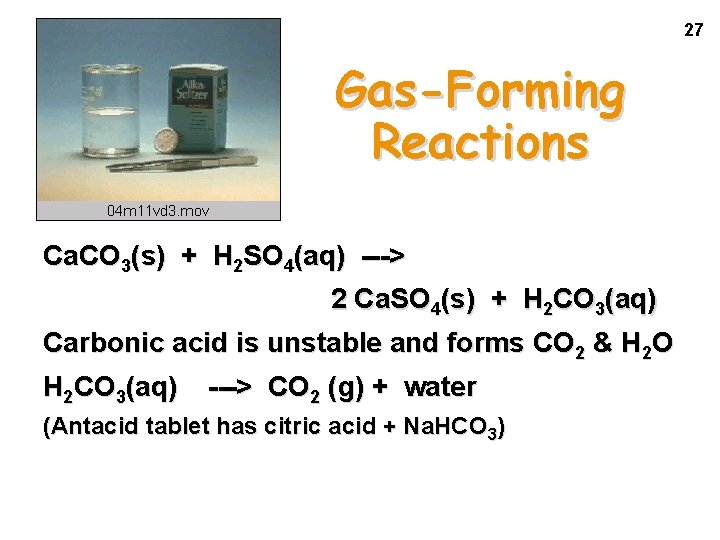
27 Gas-Forming Reactions Ca. CO 3(s) + H 2 SO 4(aq) ---> 2 Ca. SO 4(s) + H 2 CO 3(aq) Carbonic acid is unstable and forms CO 2 & H 2 O H 2 CO 3(aq) ---> CO 2 (g) + water (Antacid tablet has citric acid + Na. HCO 3)

28 See also: Gas Forming Reactions in Biological Systems Three of the pioneers in working out the roles of NO forming reactions shared a Nobel Prize in 1988 for their discoveries.

29 Quantitative Aspects of Reactions in Solution Sections 5. 8 -5. 10

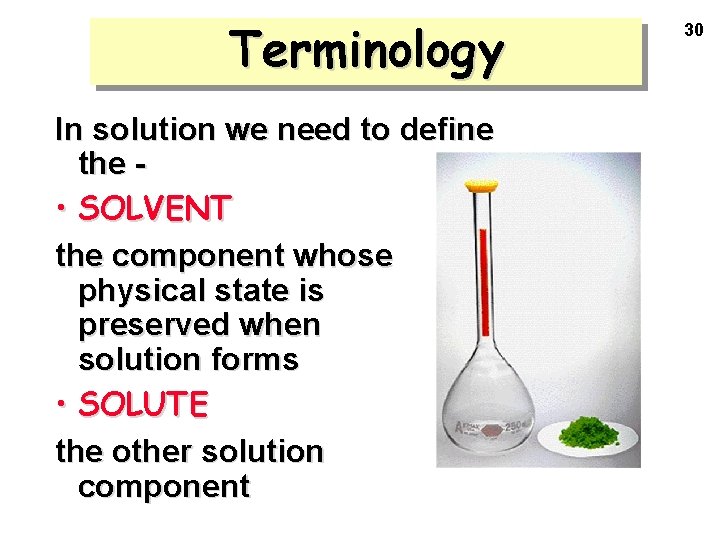
Terminology In solution we need to define the • SOLVENT the component whose physical state is preserved when solution forms • SOLUTE the other solution component 30

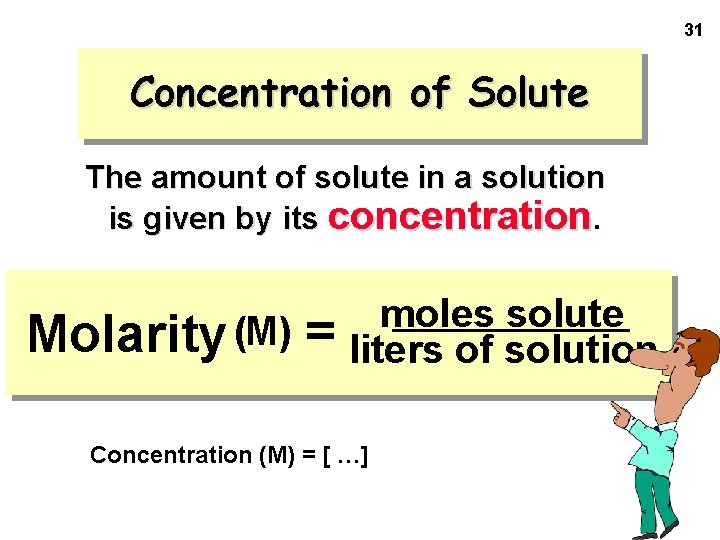
31 Concentration of Solute The amount of solute in a solution is given by its concentration. Molarity (M) = moles solute liters of solution Concentration (M) = [ …]

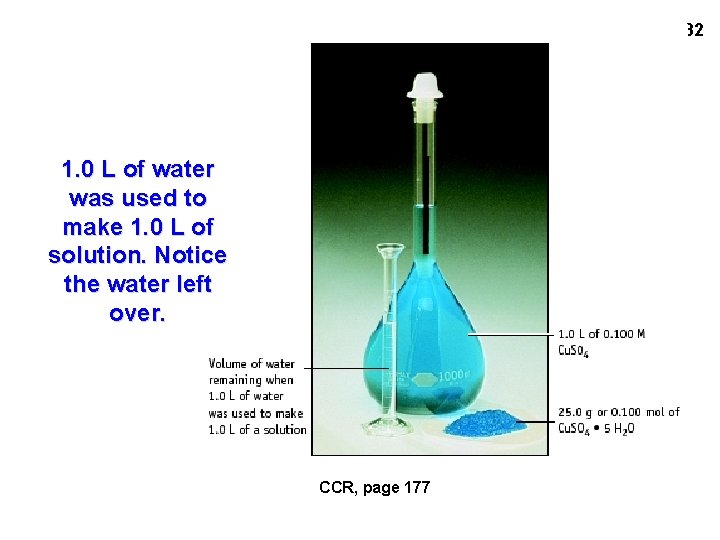
32 1. 0 L of water was used to make 1. 0 L of solution. Notice the water left over. CCR, page 177

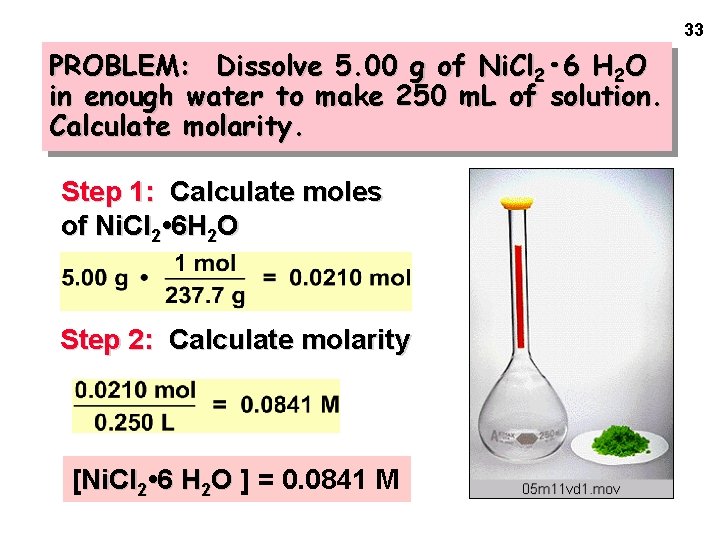
33 PROBLEM: Dissolve 5. 00 g of Ni. Cl 2 • 6 H 2 O in enough water to make 250 m. L of solution. Calculate molarity. Step 1: Calculate moles of Ni. Cl 2 • 6 H 2 O Step 2: Calculate molarity [Ni. Cl 2 • 6 H 2 O ] = 0. 0841 M

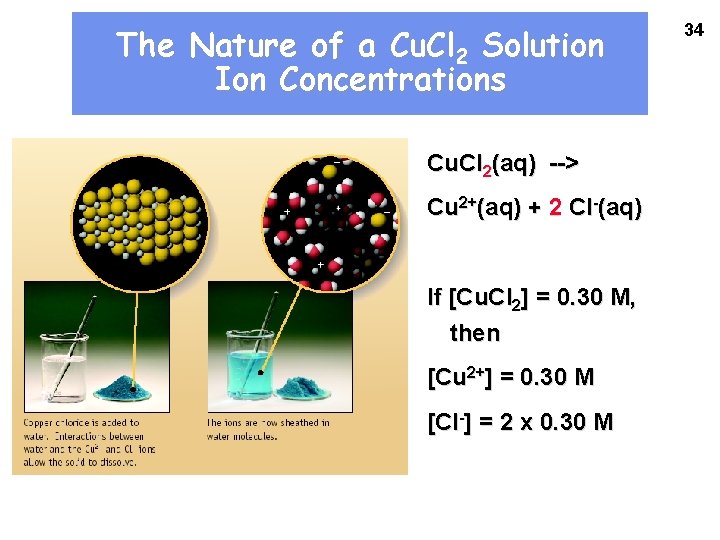
The Nature of a Cu. Cl 2 Solution Ion Concentrations Cu. Cl 2(aq) --> Cu 2+(aq) + 2 Cl-(aq) If [Cu. Cl 2] = 0. 30 M, then [Cu 2+] = 0. 30 M [Cl-] = 2 x 0. 30 M 34

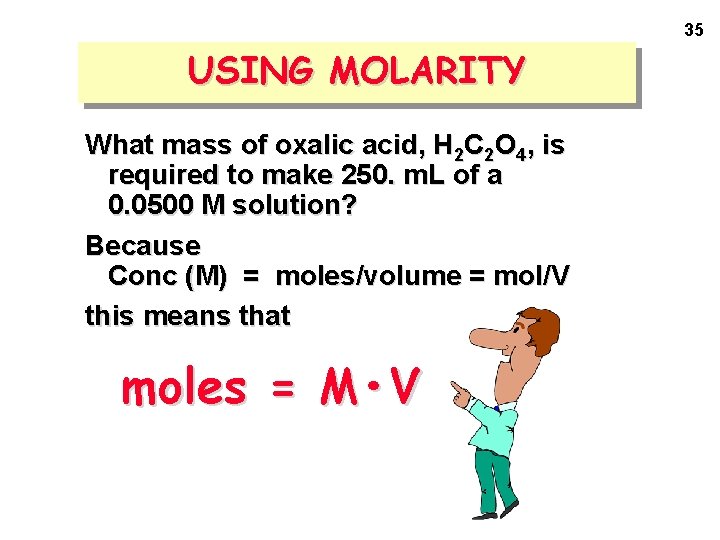
35 USING MOLARITY What mass of oxalic acid, H 2 C 2 O 4, is required to make 250. m. L of a 0. 0500 M solution? Because Conc (M) = moles/volume = mol/V this means that moles = M • V

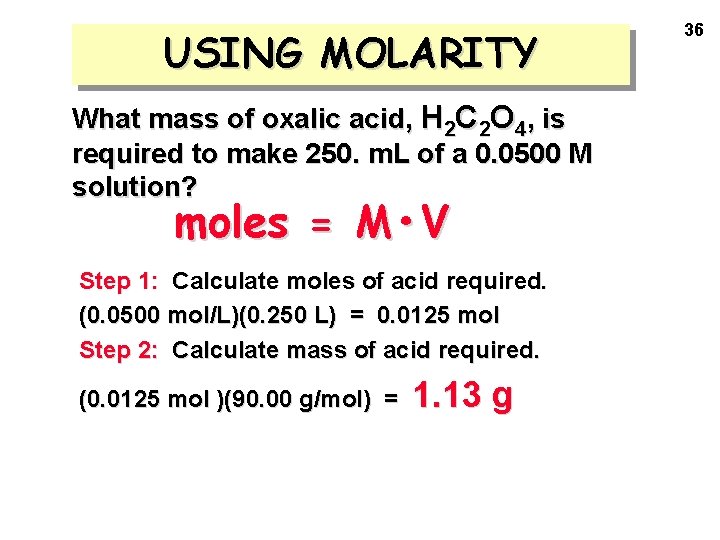
USING MOLARITY What mass of oxalic acid, H 2 C 2 O 4, is required to make 250. m. L of a 0. 0500 M solution? moles = M • V Step 1: Calculate moles of acid required. (0. 0500 mol/L)(0. 250 L) = 0. 0125 mol Step 2: Calculate mass of acid required. (0. 0125 mol )(90. 00 g/mol) = 1. 13 g 36

37 Preparing Solutions • Weigh out a solid solute and dissolve in a given quantity of solvent. • Dilute a concentrated solution to give one that is less concentrated.

38 PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? Add water to the 3. 0 M solution to lower its concentration to 0. 50 M Dilute the solution!

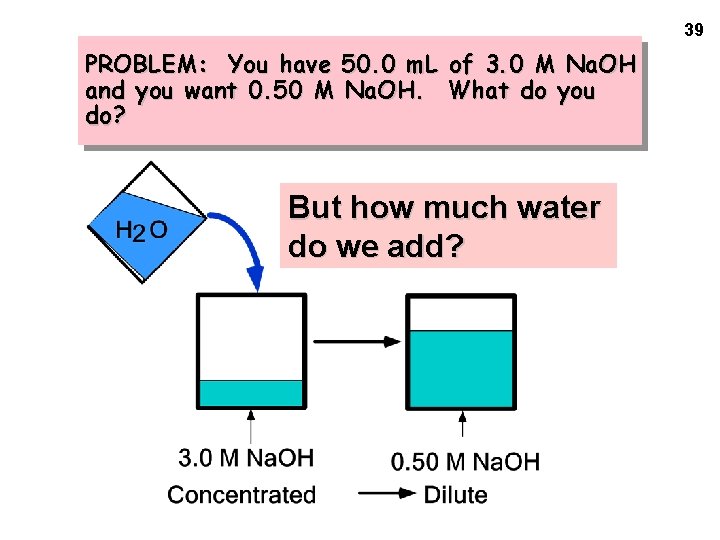
39 PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? But how much water do we add?

40 PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? How much water is added? The important point is that ---> moles of Na. OH in ORIGINAL solution = moles of Na. OH in FINAL solution

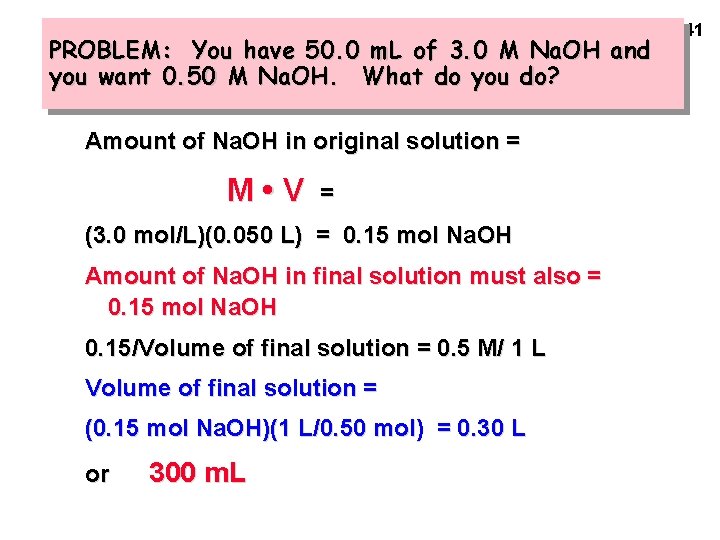
PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? Amount of Na. OH in original solution = M • V = (3. 0 mol/L)(0. 050 L) = 0. 15 mol Na. OH Amount of Na. OH in final solution must also = 0. 15 mol Na. OH 0. 15/Volume of final solution = 0. 5 M/ 1 L Volume of final solution = (0. 15 mol Na. OH)(1 L/0. 50 mol) = 0. 30 L or 300 m. L 41

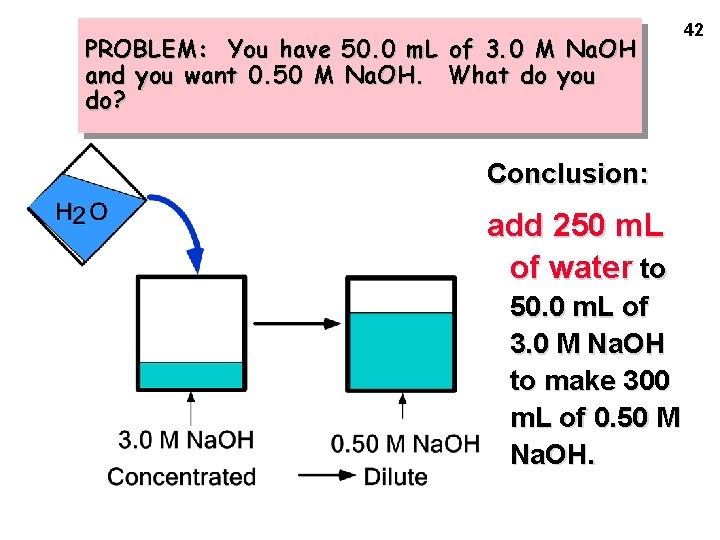
PROBLEM: You have 50. 0 m. L of 3. 0 M Na. OH and you want 0. 50 M Na. OH. What do you do? Conclusion: add 250 m. L of water to 50. 0 m. L of 3. 0 M Na. OH to make 300 m. L of 0. 50 M Na. OH. 42

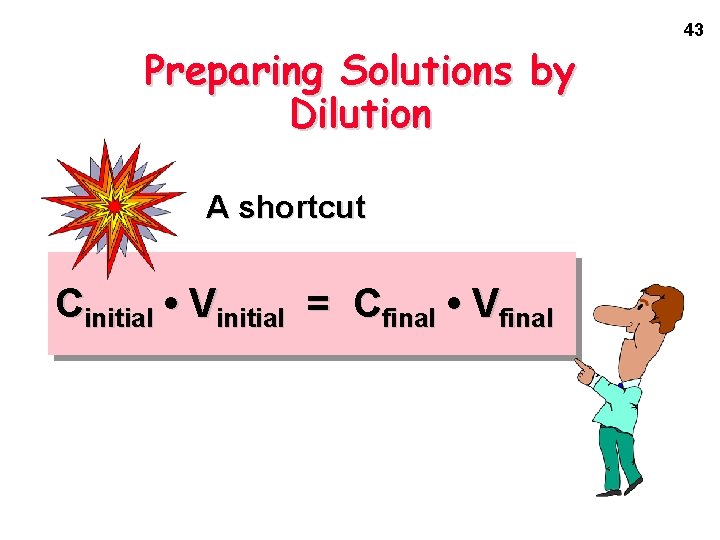
43 Preparing Solutions by Dilution A shortcut Cinitial • Vinitial = Cfinal • Vfinal

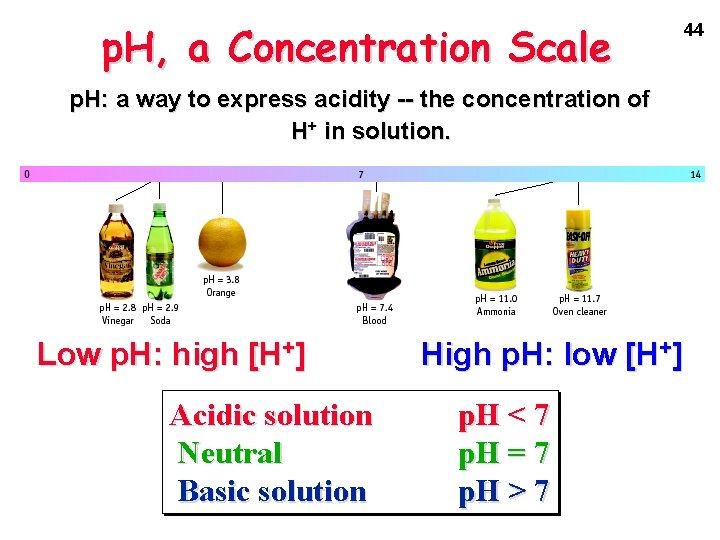
p. H, a Concentration Scale p. H: a way to express acidity -- the concentration of H+ in solution. Low p. H: high [H+] Acidic solution Neutral Basic solution High p. H: low [H+] p. H < 7 p. H = 7 p. H > 7 44
![The p H Scale 45 p H log 1 H log The p. H Scale 45 p. H = log (1/ [H+]) = - log](https://slidetodoc.com/presentation_image_h2/71242150eb11e63bd8f6c35e59871d8d/image-45.jpg)
The p. H Scale 45 p. H = log (1/ [H+]) = - log [H+] Remember : log a = b if 10 b=a In a neutral solution, [H+] = [OH-] = 1. 00 x 10 -7 M at 25 o. C p. H = - log [H+] = -log (1. 00 x 10 -7) (-7) = 7 See CD Screen 5. 17 for a tutorial = -
![H and p H If the H of soda is 1 6 x 10 [H+] and p. H If the [H+] of soda is 1. 6 x 10](https://slidetodoc.com/presentation_image_h2/71242150eb11e63bd8f6c35e59871d8d/image-46.jpg)
[H+] and p. H If the [H+] of soda is 1. 6 x 10 -3 M, the p. H is ____? Because p. H = - log [H+] then p. H= - log (1. 6 x 10 -3) p. H = - (-2. 80) p. H = 2. 80 What’s the origin of the name of the soda 7 up ? 46
![p H and H If the p H of Coke is 3 12 it p. H and [H+] If the p. H of Coke is 3. 12, it](https://slidetodoc.com/presentation_image_h2/71242150eb11e63bd8f6c35e59871d8d/image-47.jpg)
p. H and [H+] If the p. H of Coke is 3. 12, it is ______. Because p. H = - log [H+] then log [H+] = - p. H Take antilog and get + [H ] = -p. H 10 [H+] = 10 -3. 12 = 7. 6 x 10 -4 M 47

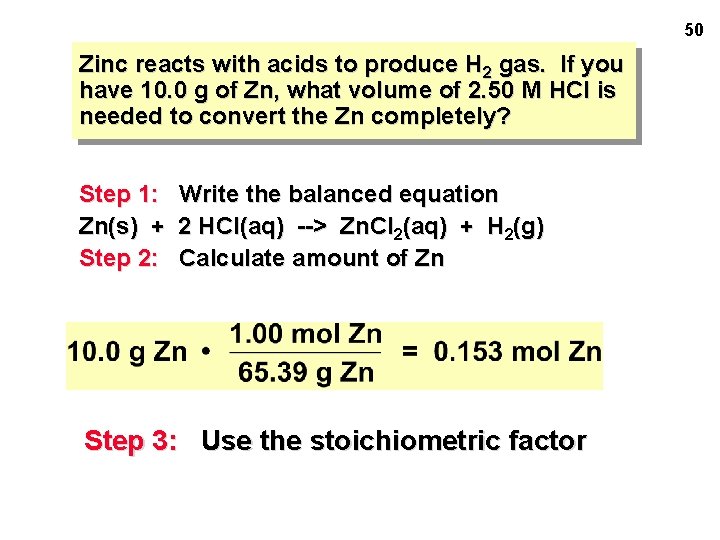
SOLUTION STOICHIOMETRY Section 5. 10 • Zinc reacts with acids to produce H 2 gas. • Have 10. 0 g of Zn • What volume of 2. 50 M HCl is needed to convert the Zn completely? 48

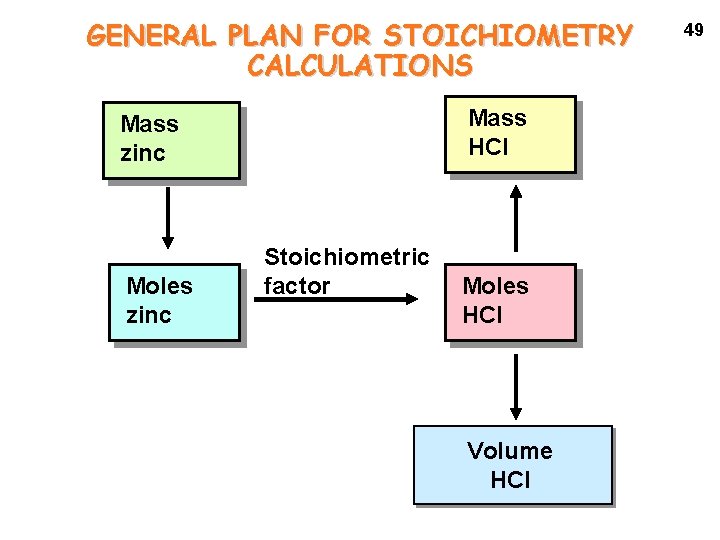
GENERAL PLAN FOR STOICHIOMETRY CALCULATIONS Mass HCl Mass zinc Moles zinc Stoichiometric factor Moles HCl Volume HCl 49

50 Zinc reacts with acids to produce H 2 gas. If you have 10. 0 g of Zn, what volume of 2. 50 M HCl is needed to convert the Zn completely? Step 1: Write the balanced equation Zn(s) + 2 HCl(aq) --> Zn. Cl 2(aq) + H 2(g) Step 2: Calculate amount of Zn Step 3: Use the stoichiometric factor

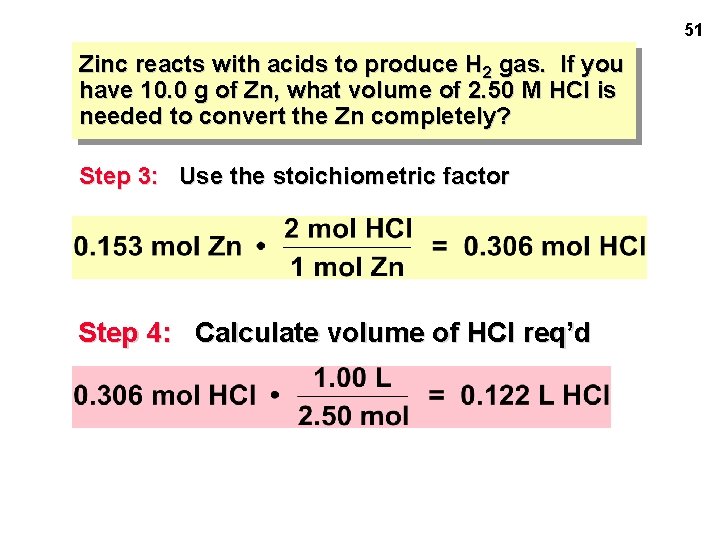
51 Zinc reacts with acids to produce H 2 gas. If you have 10. 0 g of Zn, what volume of 2. 50 M HCl is needed to convert the Zn completely? Step 3: Use the stoichiometric factor Step 4: Calculate volume of HCl req’d

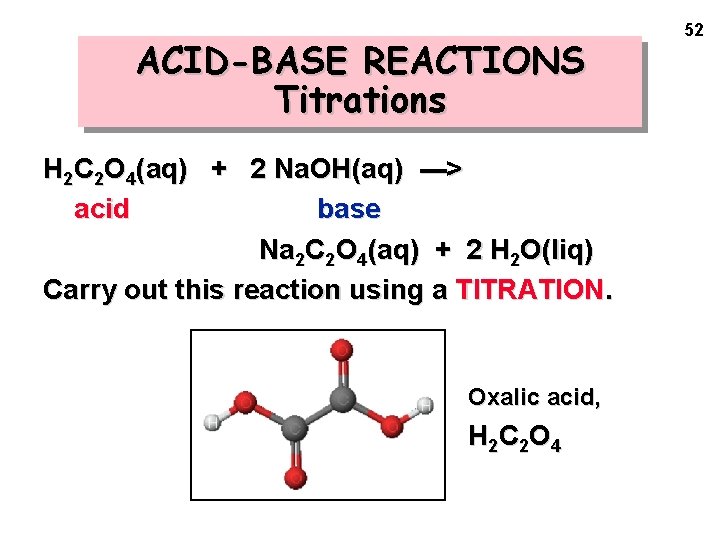
ACID-BASE REACTIONS Titrations H 2 C 2 O 4(aq) + 2 Na. OH(aq) ---> acid base Na 2 C 2 O 4(aq) + 2 H 2 O(liq) Carry out this reaction using a TITRATION. Oxalic acid, H 2 C 2 O 4 52

Setup for titrating an acid with a base CCR, page 186 53

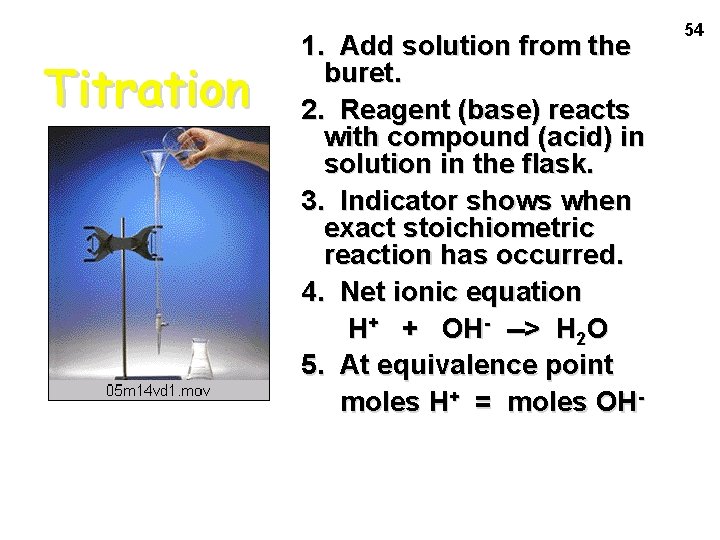
Titration 1. Add solution from the buret. 2. Reagent (base) reacts with compound (acid) in solution in the flask. 3. Indicator shows when exact stoichiometric reaction has occurred. 4. Net ionic equation H+ + OH- --> H 2 O 5. At equivalence point moles H+ = moles OH- 54

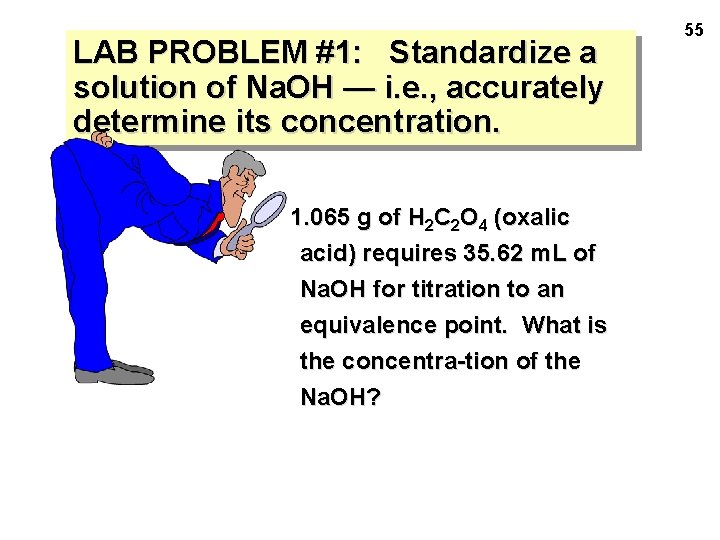
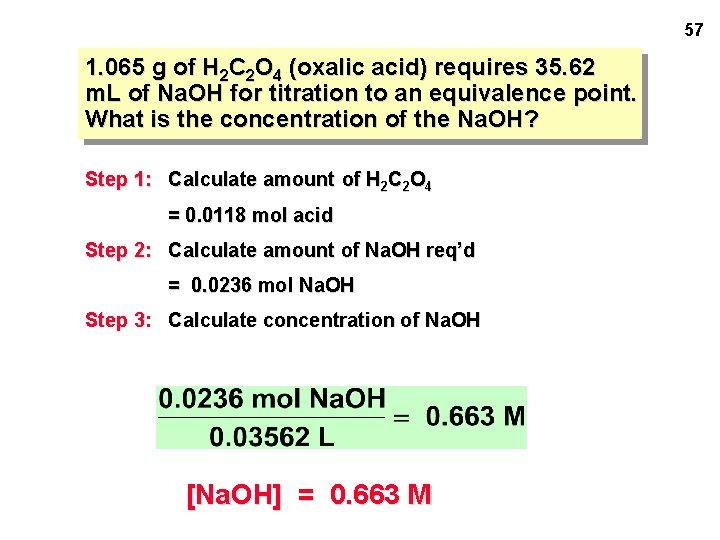
LAB PROBLEM #1: Standardize a solution of Na. OH — i. e. , accurately determine its concentration. 1. 065 g of H 2 C 2 O 4 (oxalic acid) requires 35. 62 m. L of Na. OH for titration to an equivalence point. What is the concentra-tion of the Na. OH? 55

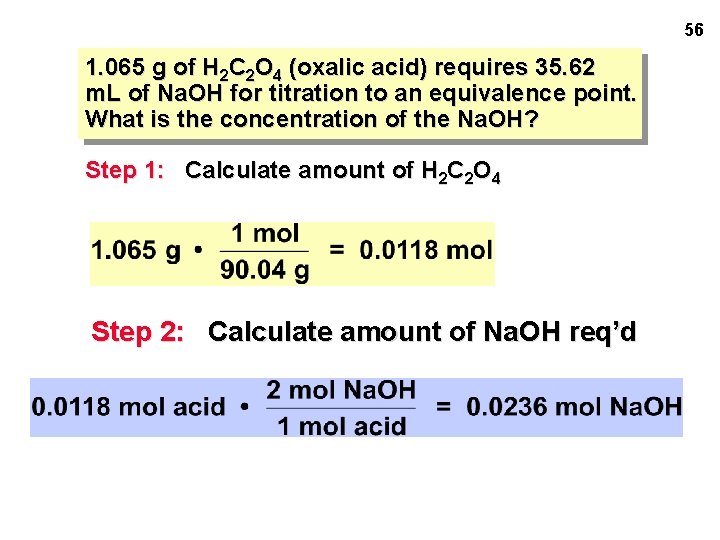
56 1. 065 g of H 2 C 2 O 4 (oxalic acid) requires 35. 62 m. L of Na. OH for titration to an equivalence point. What is the concentration of the Na. OH? Step 1: Calculate amount of H 2 C 2 O 4 Step 2: Calculate amount of Na. OH req’d

57 1. 065 g of H 2 C 2 O 4 (oxalic acid) requires 35. 62 m. L of Na. OH for titration to an equivalence point. What is the concentration of the Na. OH? Step 1: Calculate amount of H 2 C 2 O 4 = 0. 0118 mol acid Step 2: Calculate amount of Na. OH req’d = 0. 0236 mol Na. OH Step 3: Calculate concentration of Na. OH [Na. OH] = 0. 663 M

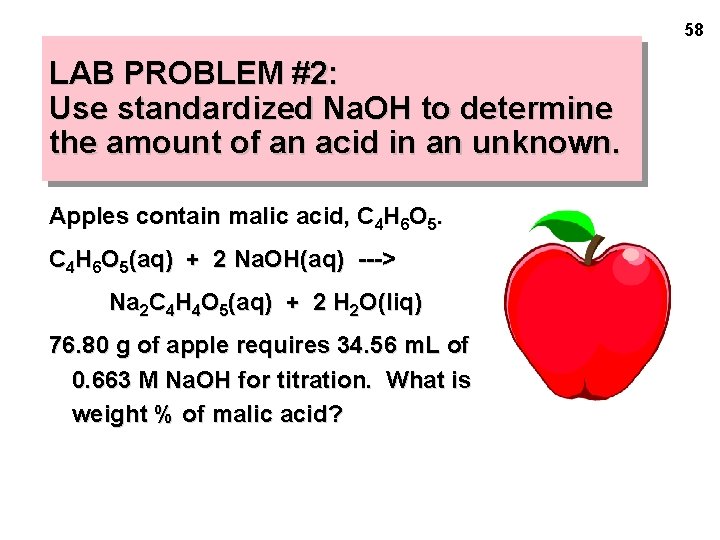
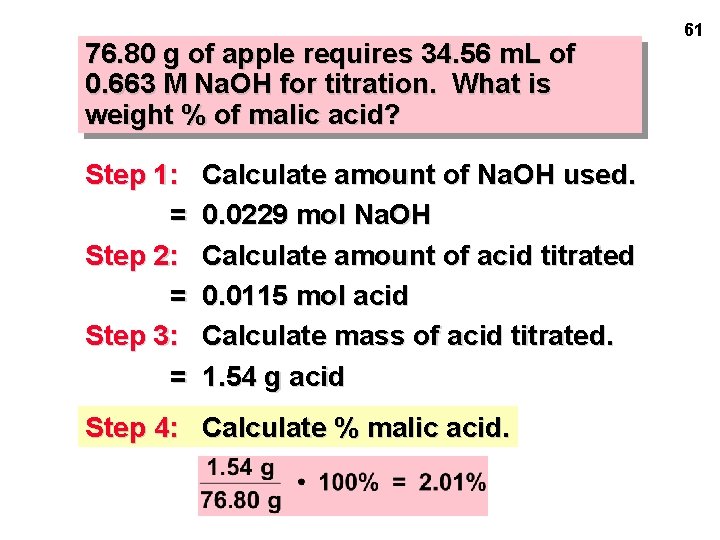
58 LAB PROBLEM #2: Use standardized Na. OH to determine the amount of an acid in an unknown. Apples contain malic acid, C 4 H 6 O 5(aq) + 2 Na. OH(aq) ---> Na 2 C 4 H 4 O 5(aq) + 2 H 2 O(liq) 76. 80 g of apple requires 34. 56 m. L of 0. 663 M Na. OH for titration. What is weight % of malic acid?

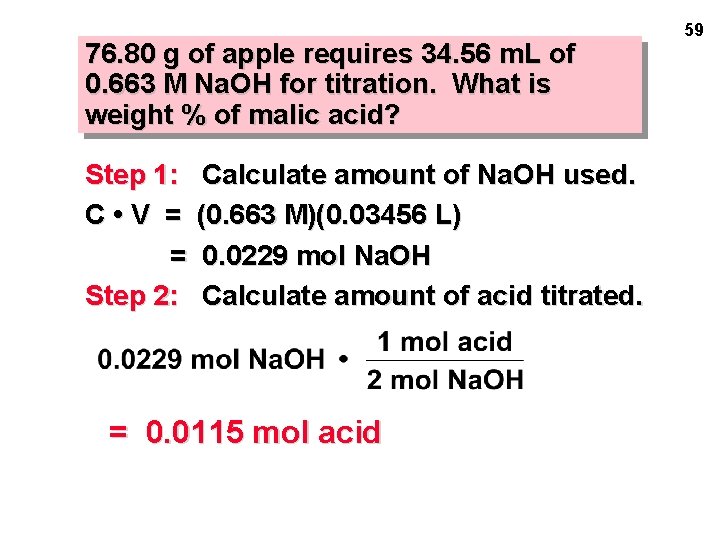
76. 80 g of apple requires 34. 56 m. L of 0. 663 M Na. OH for titration. What is weight % of malic acid? Step 1: C • V = = Step 2: Calculate amount of Na. OH used. (0. 663 M)(0. 03456 L) 0. 0229 mol Na. OH Calculate amount of acid titrated. = 0. 0115 mol acid 59

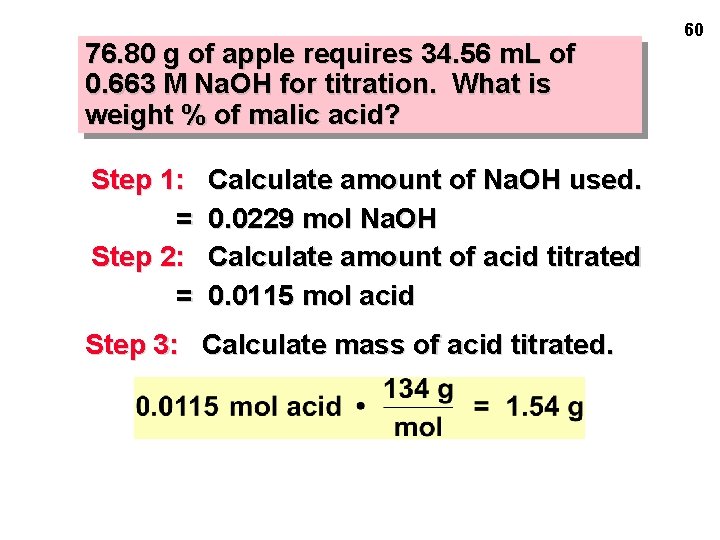
76. 80 g of apple requires 34. 56 m. L of 0. 663 M Na. OH for titration. What is weight % of malic acid? Step 1: = Step 2: = Calculate amount of Na. OH used. 0. 0229 mol Na. OH Calculate amount of acid titrated 0. 0115 mol acid Step 3: Calculate mass of acid titrated. 60

76. 80 g of apple requires 34. 56 m. L of 0. 663 M Na. OH for titration. What is weight % of malic acid? Step 1: = Step 2: = Step 3: = Calculate amount of Na. OH used. 0. 0229 mol Na. OH Calculate amount of acid titrated 0. 0115 mol acid Calculate mass of acid titrated. 1. 54 g acid Step 4: Calculate % malic acid. 61

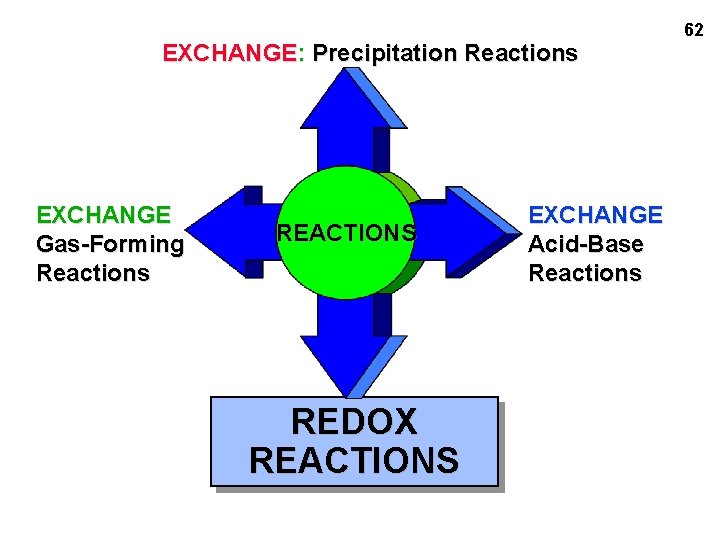
EXCHANGE: Precipitation Reactions EXCHANGE Gas-Forming Reactions REACTIONS REDOX REACTIONS EXCHANGE Acid-Base Reactions 62

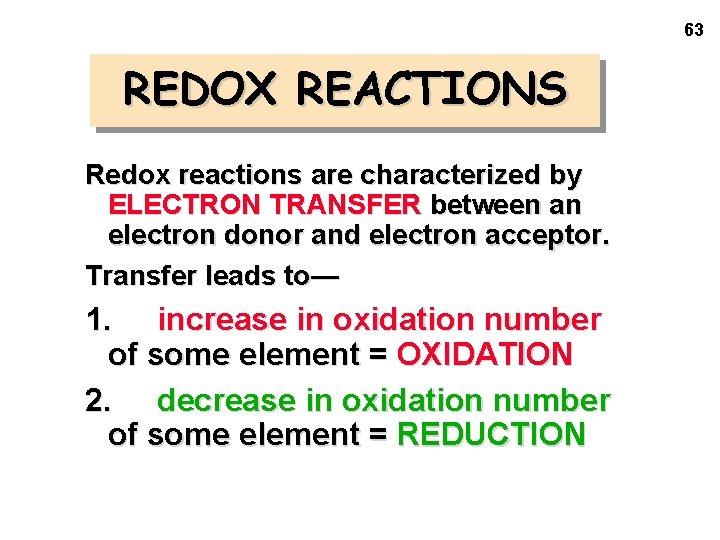
63 REDOX REACTIONS Redox reactions are characterized by ELECTRON TRANSFER between an electron donor and electron acceptor. Transfer leads to— 1. increase in oxidation number of some element = OXIDATION 2. decrease in oxidation number of some element = REDUCTION

64 REDOX REACTIONS Cu(s) + 2 Ag+(aq) ---> Cu 2+(aq) + 2 Ag(s) In all reactions if something has been oxidized then something has also been reduced

65 Why Study Redox Reactions Batteries Corrosion Manufacturing metals Fuels

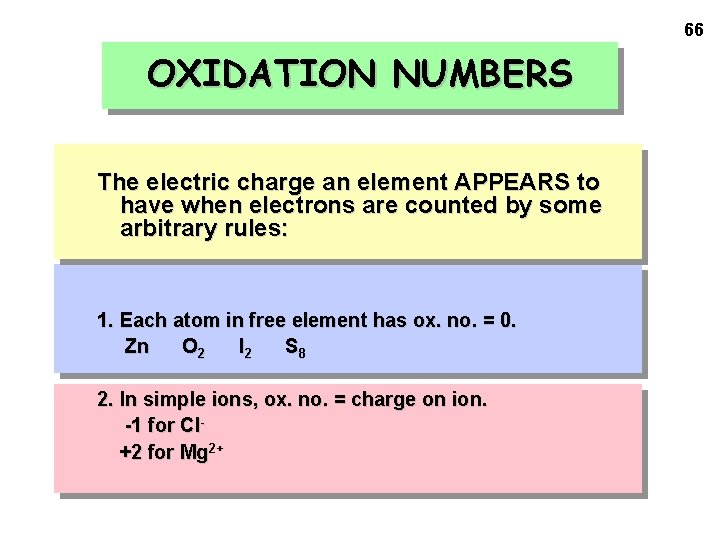
66 OXIDATION NUMBERS The electric charge an element APPEARS to have when electrons are counted by some arbitrary rules: 1. Each atom in free element has ox. no. = 0. Zn O 2 I 2 S 8 2. In simple ions, ox. no. = charge on ion. -1 for Cl+2 for Mg 2+

OXIDATION NUMBERS 3. F always has an oxidation number of -1 when forming compounds with other elements. 4. Cl, Br and I have oxidation numbers of -1 when forming compounds with other elements, except when combined with oxygen and fluorine. 5 a. O has ox. no. = -2 (except in peroxides: in H 2 O 2, O = -1) 67

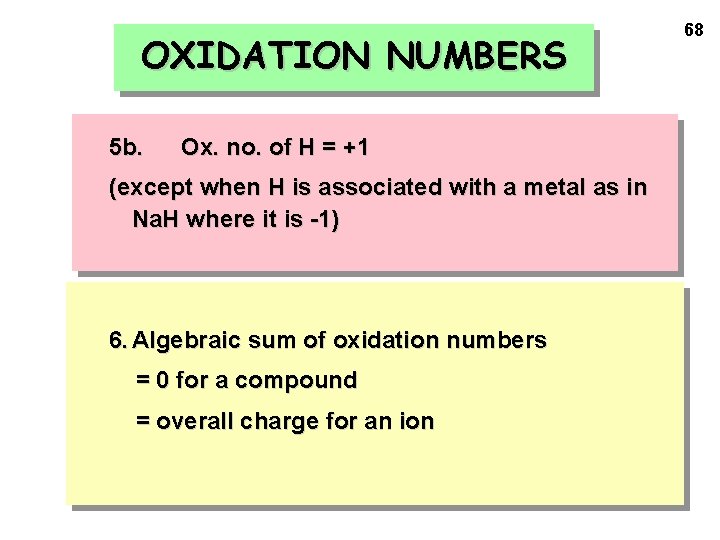
OXIDATION NUMBERS 5 b. Ox. no. of H = +1 (except when H is associated with a metal as in Na. H where it is -1) 6. Algebraic sum of oxidation numbers = 0 for a compound = overall charge for an ion 68

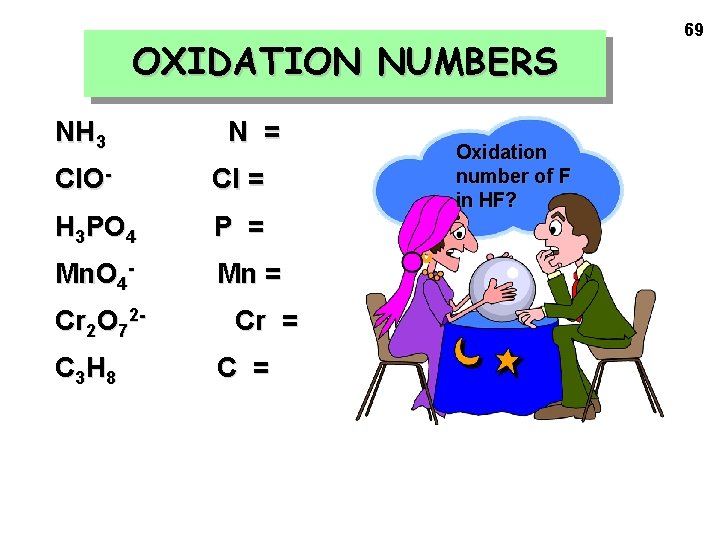
OXIDATION NUMBERS NH 3 N = Cl. O- Cl = H 3 PO 4 P = Mn. O 4 - Mn = Cr 2 O 72 C 3 H 8 Cr = C = Oxidation number of F in HF? 69

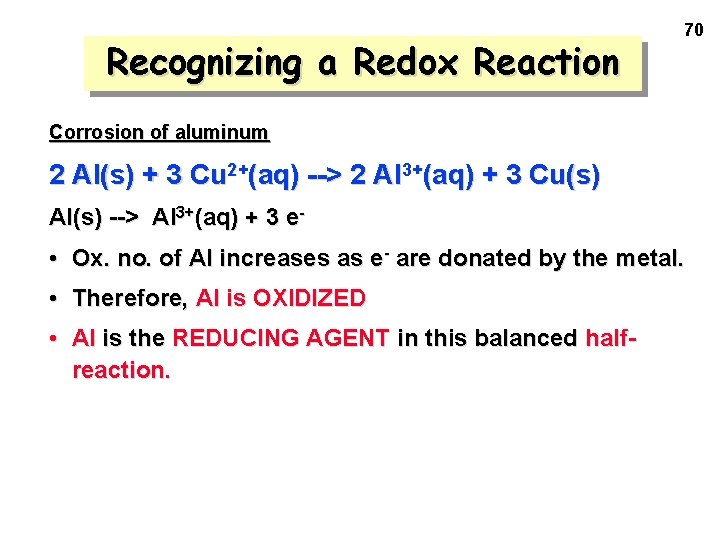
Recognizing a Redox Reaction 70 Corrosion of aluminum 2 Al(s) + 3 Cu 2+(aq) --> 2 Al 3+(aq) + 3 Cu(s) Al(s) --> Al 3+(aq) + 3 e- • Ox. no. of Al increases as e- are donated by the metal. • Therefore, Al is OXIDIZED • Al is the REDUCING AGENT in this balanced halfreaction.

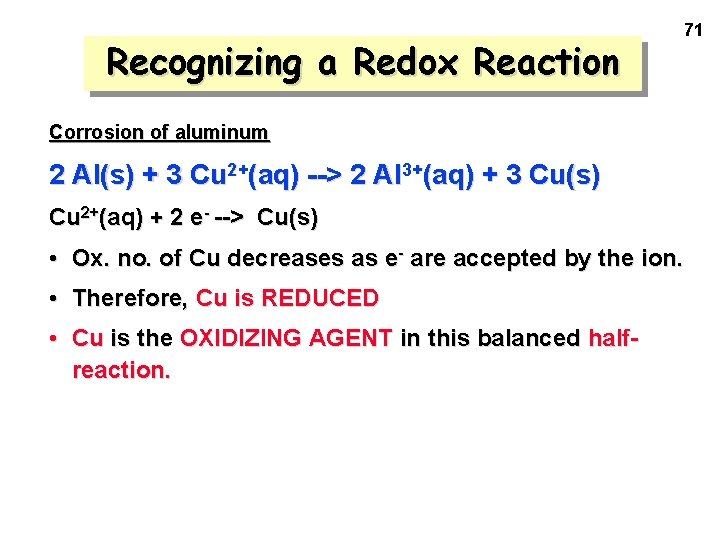
Recognizing a Redox Reaction 71 Corrosion of aluminum 2 Al(s) + 3 Cu 2+(aq) --> 2 Al 3+(aq) + 3 Cu(s) Cu 2+(aq) + 2 e- --> Cu(s) • Ox. no. of Cu decreases as e- are accepted by the ion. • Therefore, Cu is REDUCED • Cu is the OXIDIZING AGENT in this balanced halfreaction.

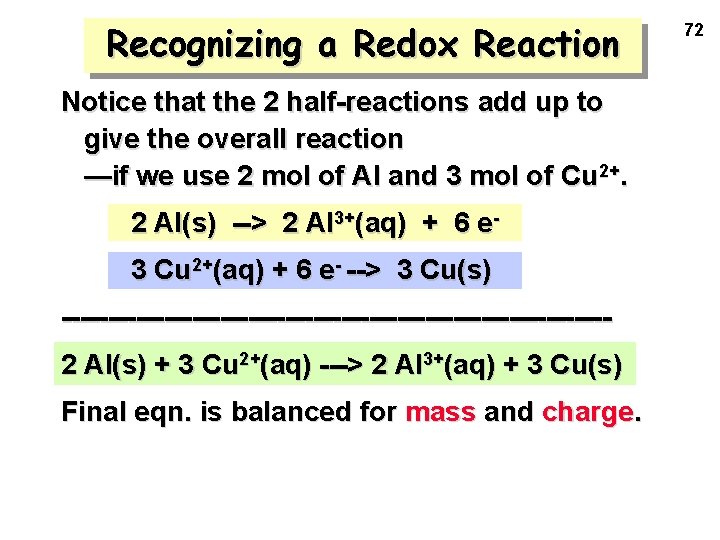
Recognizing a Redox Reaction Notice that the 2 half-reactions add up to give the overall reaction —if we use 2 mol of Al and 3 mol of Cu 2+. 2 Al(s) --> 2 Al 3+(aq) + 6 e 3 Cu 2+(aq) + 6 e- --> 3 Cu(s) -----------------------------2 Al(s) + 3 Cu 2+(aq) ---> 2 Al 3+(aq) + 3 Cu(s) Final eqn. is balanced for mass and charge. 72

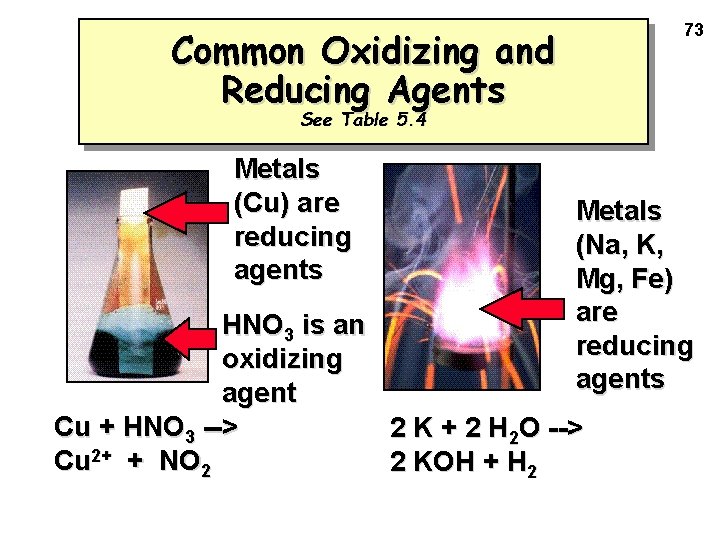
73 Common Oxidizing and Reducing Agents See Table 5. 4 Metals (Cu) are reducing agents Metals (Na, K, Mg, Fe) are reducing agents HNO 3 is an oxidizing agent Cu + HNO 3 --> 2 K + 2 H 2 O --> Cu 2+ + NO 2 2 KOH + H 2

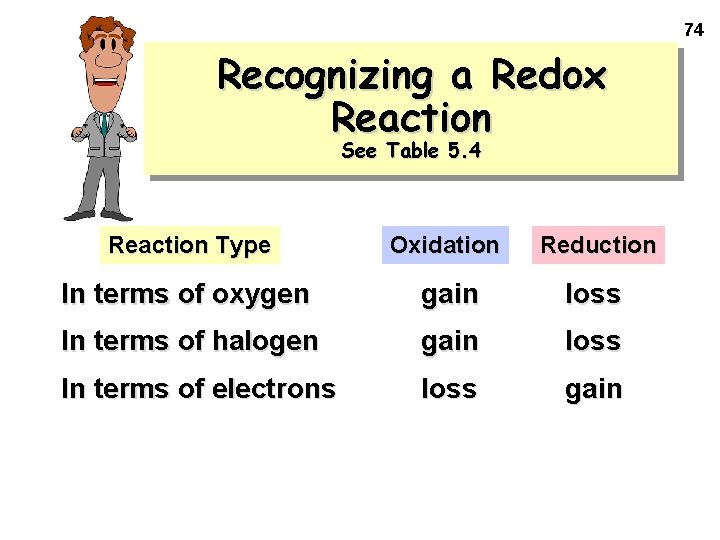
74 Recognizing a Redox Reaction See Table 5. 4 Reaction Type Oxidation Reduction In terms of oxygen gain loss In terms of halogen gain loss In terms of electrons loss gain

Examples of Redox Reactions Metal + halogen 2 Al + 3 Br 2 ---> Al 2 Br 6 75

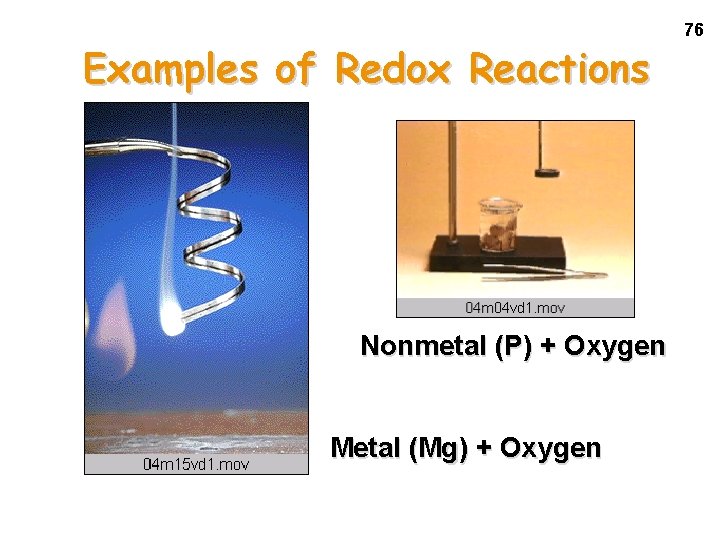
Examples of Redox Reactions Nonmetal (P) + Oxygen Metal (Mg) + Oxygen 76

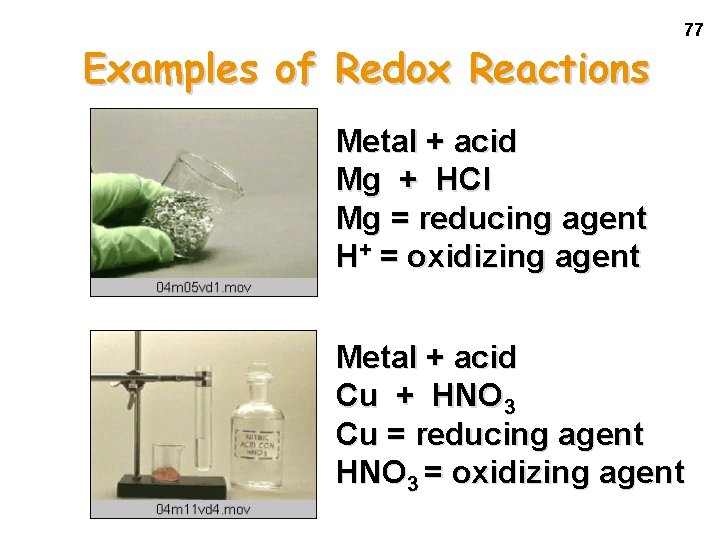
Examples of Redox Reactions 77 Metal + acid Mg + HCl Mg = reducing agent H+ = oxidizing agent Metal + acid Cu + HNO 3 Cu = reducing agent HNO 3 = oxidizing agent