Ionic Compounds Involving Transition Metals Formulas Nomenclature What











- Slides: 15

Ionic Compounds Involving Transition Metals Formulas & Nomenclature

What Are They? • Transition metals are those metals whose valence electrons can have more than one orientation. • This allows them to have multiple oxidation numbers – multiple charges. • We must take this into consideration when naming transition metal ionic compounds

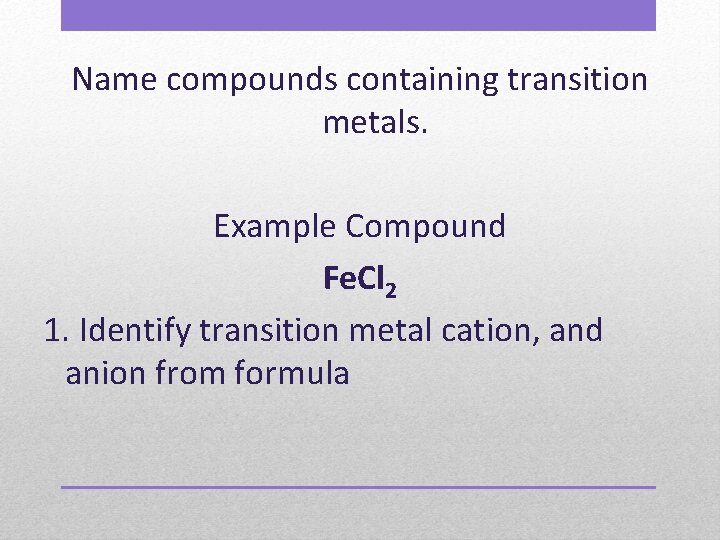
Name compounds containing transition metals. Example Compound Fe. Cl 2 1. Identify transition metal cation, and anion from formula

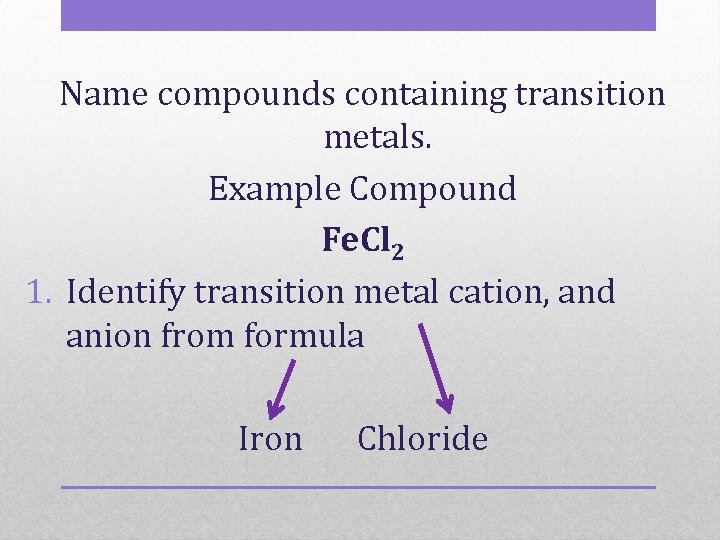
Name compounds containing transition metals. Example Compound Fe. Cl 2 1. Identify transition metal cation, and anion from formula Iron Chloride

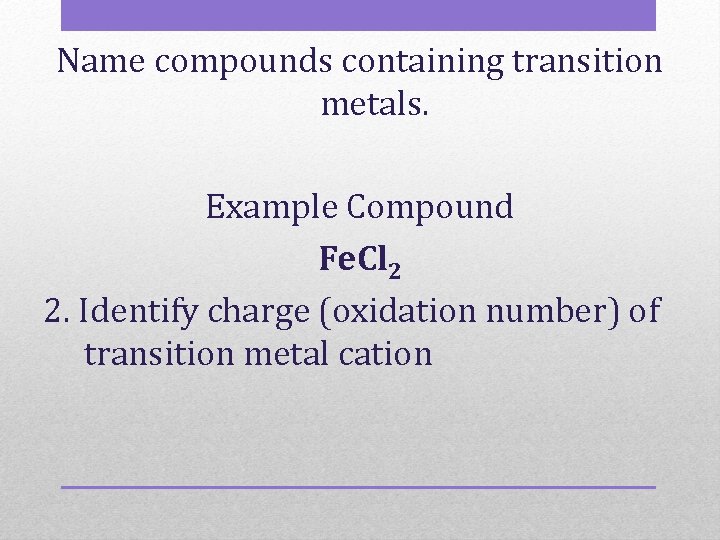
Name compounds containing transition metals. Example Compound Fe. Cl 2 2. Identify charge (oxidation number) of transition metal cation

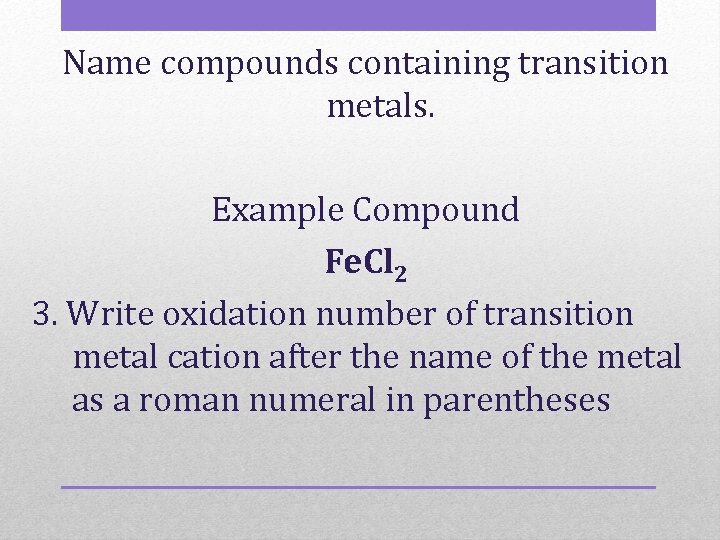
Name compounds containing transition metals. Example Compound Fe. Cl 2 3. Write oxidation number of transition metal cation after the name of the metal as a roman numeral in parentheses

Example Compound Fe. Cl 2 Iron (II) Chloride

Name the following transition metal ionic compounds 1. Ni. Cl 2 2. Cu 2 O 3. Sn. Br 4

Given the name of a compound containing a transition metal, write its formula. Example Name: Cobalt (III) Chloride 1. Determine charge of transition metal cation 3+ Co

Given the name of a compound containing a transition metal, write its formula. Example Name: Cobalt (III) Chloride 2. Determine charge of individual anion

Given the name of a compound containing a transition metal, write its formula. Example Name: Cobalt (III) Chloride 2. Determine charge of individual anion Chloride anion has a -1 charge

Given the name of a compound containing a transition metal, write its formula. Example Name: Cobalt (III) Chloride 3. Determine ratio of cations and anions needed to balance the charge

Given the name of a compound containing a transition metal, write its formula. Example Name: Cobalt (III) Chloride 3. Determine ratio of cations and anions needed to balance the charge 1 Co 3+ to 3 Cl -

Cobalt (III) Chloride Co. Cl 3

Give formulas for the following compounds using their names 1. Chromium (III) Sulfide 2. Mercury (I) Chloride 3. Zinc (II) Nitride