Ionic compounds contain positive and negative ions They


























- Slides: 64


• Ionic compounds contain positive and negative ions. They are held together by electrostatic attraction. Most of the negative ions have only one possible oxidation state. This is not the case with the positive ions. Many of these ions have several oxidation states. The use of prefixes in naming these compounds could lead to confusion. Consequently a new method “the stock system” is very specific in the naming of ionic compounds.

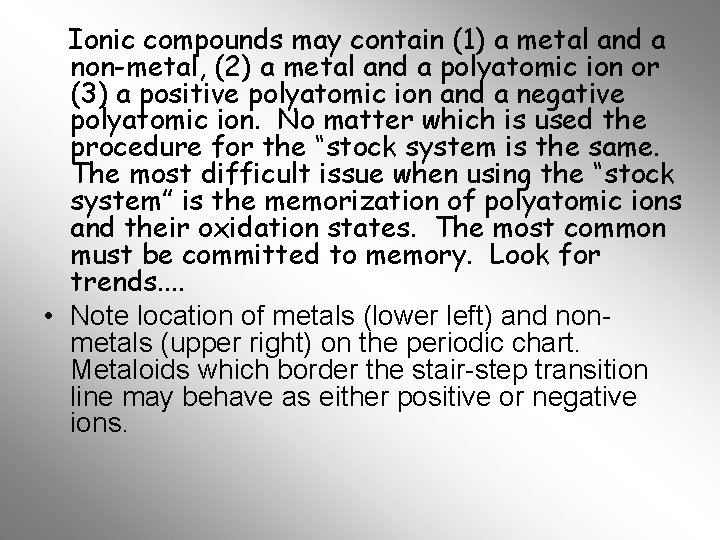
Ionic compounds may contain (1) a metal and a non-metal, (2) a metal and a polyatomic ion or (3) a positive polyatomic ion and a negative polyatomic ion. No matter which is used the procedure for the “stock system is the same. The most difficult issue when using the “stock system” is the memorization of polyatomic ions and their oxidation states. The most common must be committed to memory. Look for trends. . • Note location of metals (lower left) and nonmetals (upper right) on the periodic chart. Metaloids which border the stair-step transition line may behave as either positive or negative ions.

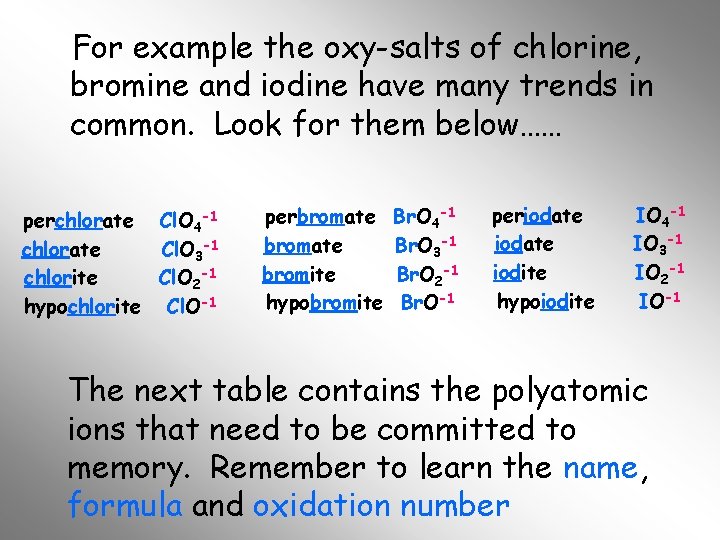
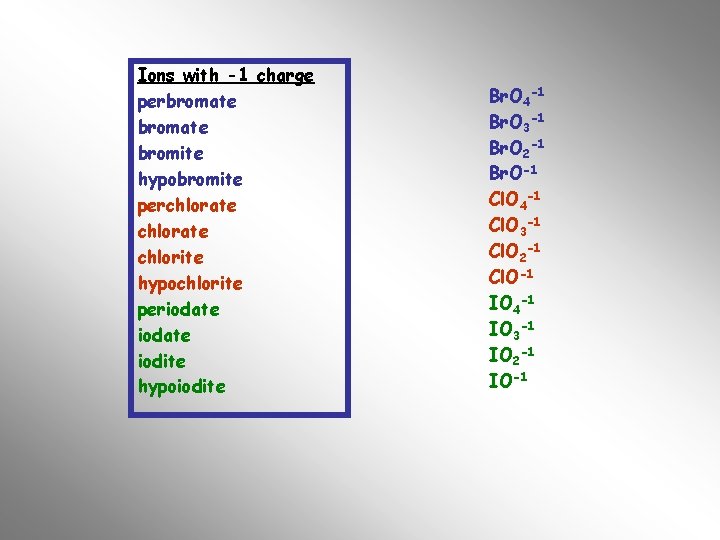
For example the oxy-salts of chlorine, bromine and iodine have many trends in common. Look for them below…… perchlorate Cl. O 4 -1 chlorate Cl. O 3 -1 chlorite Cl. O 2 -1 hypochlorite Cl. O-1 perbromate bromite hypobromite Br. O 4 -1 Br. O 3 -1 Br. O 2 -1 Br. O-1 periodate iodite hypoiodite IO 4 -1 IO 3 -1 IO 2 -1 IO-1 The next table contains the polyatomic ions that need to be committed to memory. Remember to learn the name, formula and oxidation number

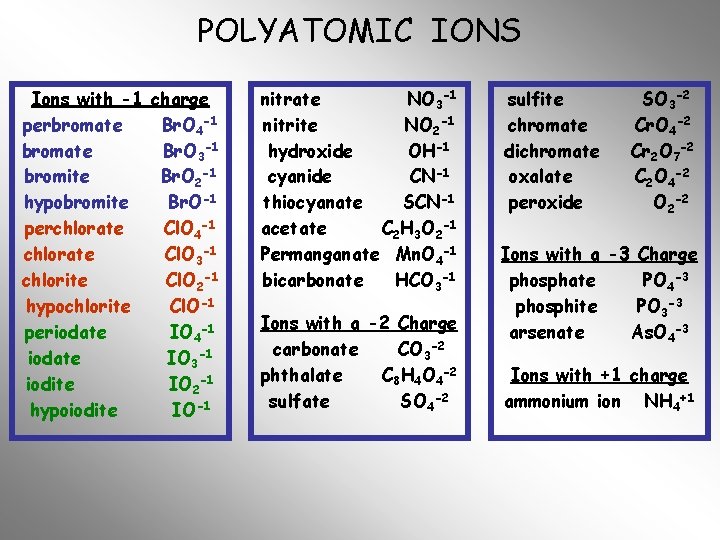
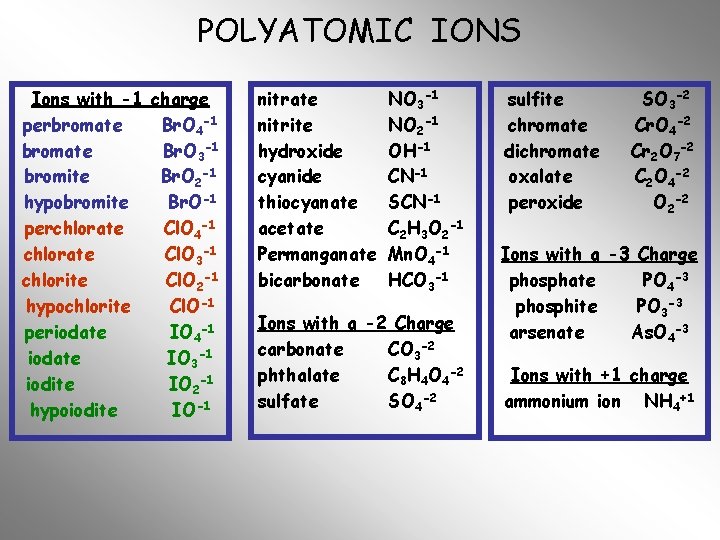
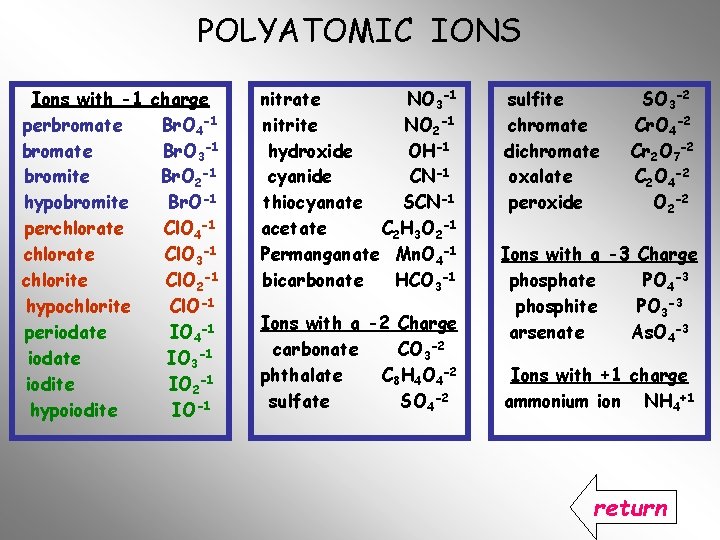
POLYATOMIC IONS Ions with -1 charge perbromate Br. O 4 -1 bromate Br. O 3 -1 bromite Br. O 2 -1 hypobromite Br. O-1 perchlorate Cl. O 4 -1 chlorate Cl. O 3 -1 chlorite Cl. O 2 -1 hypochlorite Cl. O-1 periodate IO 4 -1 iodate IO 3 -1 iodite IO 2 -1 hypoiodite IO-1 nitrate NO 3 -1 nitrite NO 2 -1 hydroxide OH-1 cyanide CN-1 thiocyanate SCN-1 acetate C 2 H 3 O 2 -1 Permanganate Mn. O 4 -1 bicarbonate HCO 3 -1 Ions with a -2 Charge carbonate CO 3 -2 phthalate C 8 H 4 O 4 -2 sulfate SO 4 -2 sulfite chromate dichromate oxalate peroxide SO 3 -2 Cr. O 4 -2 Cr 2 O 7 -2 C 2 O 4 -2 O 2 -2 Ions with a -3 Charge phosphate PO 4 -3 phosphite PO 3 -3 arsenate As. O 4 -3 Ions with +1 charge ammonium ion NH 4+1

POLYATOMIC IONS Ions with -1 charge perbromate Br. O 4 -1 bromate Br. O 3 -1 bromite Br. O 2 -1 hypobromite Br. O-1 perchlorate Cl. O 4 -1 chlorate Cl. O 3 -1 chlorite Cl. O 2 -1 hypochlorite Cl. O-1 periodate IO 4 -1 iodate IO 3 -1 iodite IO 2 -1 hypoiodite IO-1 nitrate nitrite hydroxide cyanide thiocyanate acetate Permanganate bicarbonate NO 3 -1 NO 2 -1 OH-1 CN-1 SCN-1 C 2 H 3 O 2 -1 Mn. O 4 -1 HCO 3 -1 Ions with a -2 Charge carbonate CO 3 -2 phthalate C 8 H 4 O 4 -2 sulfate SO 4 -2 sulfite chromate dichromate oxalate peroxide SO 3 -2 Cr. O 4 -2 Cr 2 O 7 -2 C 2 O 4 -2 O 2 -2 Ions with a -3 Charge phosphate PO 4 -3 phosphite PO 3 -3 arsenate As. O 4 -3 Ions with +1 charge ammonium ion NH 4+1

Ions with -1 charge perbromate bromite hypobromite perchlorate chlorite hypochlorite periodate iodite hypoiodite Br. O 4 -1 Br. O 3 -1 Br. O 2 -1 Br. O-1 Cl. O 4 -1 Cl. O 3 -1 Cl. O 2 -1 Cl. O-1 IO 4 -1 IO 3 -1 IO 2 -1 IO-1

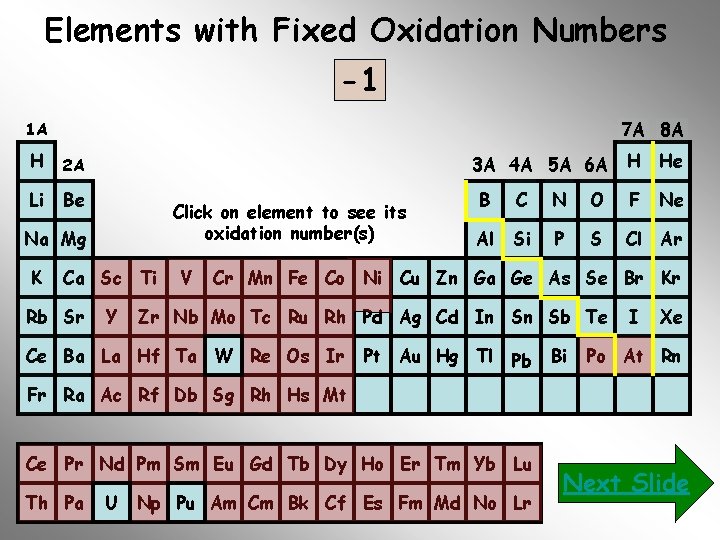
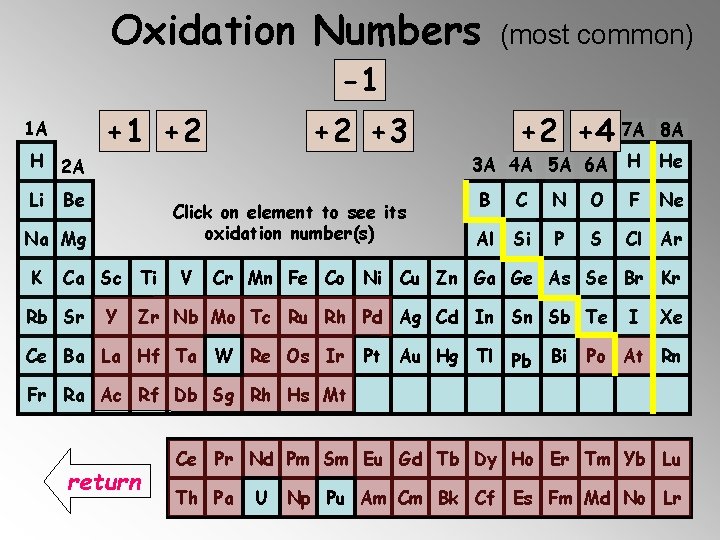
It is also important to memorize the oxidation of the monatomic ions that have fixed oxidation numbers (positive or negative). In particular, those from groups 1 A, 2 A, 3 A, 4 A, 5 A, 6 A, 7 A, 8 A and the elements: Zn, Cd and Ag. Use the following periodic chart to determine their oxidation numbers. Just click on the element symbol…

Elements with Fixed Oxidation Numbers -1 -2 -3 -4 +3 +2 +1 0 7 A 8 A 1 A H 2 A Li Be 3 A 4 A 5 A 6 A Click on element to see its oxidation number(s) Na Mg K Ca Sc Ti Rb Sr Y V H He B C N O F Ne Al Si P S Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Ce Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi I Xe Po At Rn Fr Ra Ac Rf Db Sg Rh Hs Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Next Slide

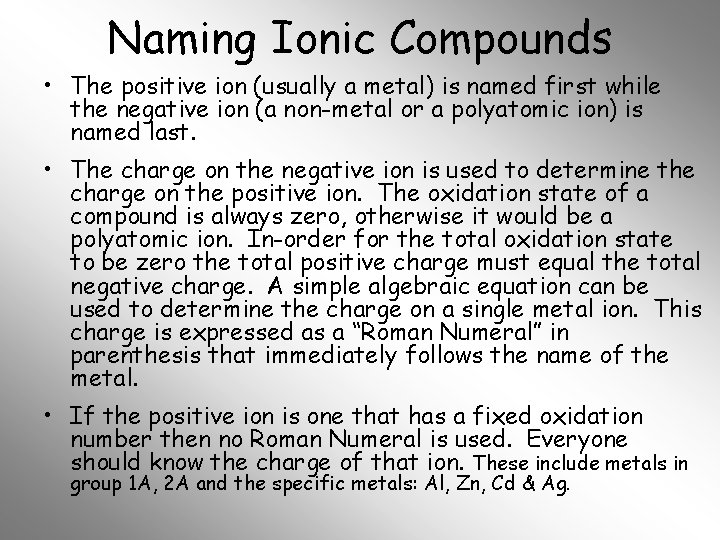
Naming Ionic Compounds • The positive ion (usually a metal) is named first while the negative ion (a non-metal or a polyatomic ion) is named last. • The charge on the negative ion is used to determine the charge on the positive ion. The oxidation state of a compound is always zero, otherwise it would be a polyatomic ion. In-order for the total oxidation state to be zero the total positive charge must equal the total negative charge. A simple algebraic equation can be used to determine the charge on a single metal ion. This charge is expressed as a “Roman Numeral” in parenthesis that immediately follows the name of the metal. • If the positive ion is one that has a fixed oxidation number then no Roman Numeral is used. Everyone should know the charge of that ion. These include metals in group 1 A, 2 A and the specific metals: Al, Zn, Cd & Ag.

• If the ionic compound is binary it will end in “-ide”. However, not all compounds that end in “-ide” are binary. For example sodium hydroxide has the formula…Na. OH (three different kinds of atoms). • If the negative ion is a polyatomic ion the compound is no longer binary. The ending will be that carried by the polyatomic ion. These endings are either “-ate” or “-ite. ” • Hydrated compounds are named using a combination of both the stock system and prefixes. A prefix is used to denote the number of water molecules attached to the ionic formula. “hydrate” is used as the name indicating that water is attached. For example, copper(II) sulfate pentahydrate has the formula Cu. SO 4. 5 H 2 O

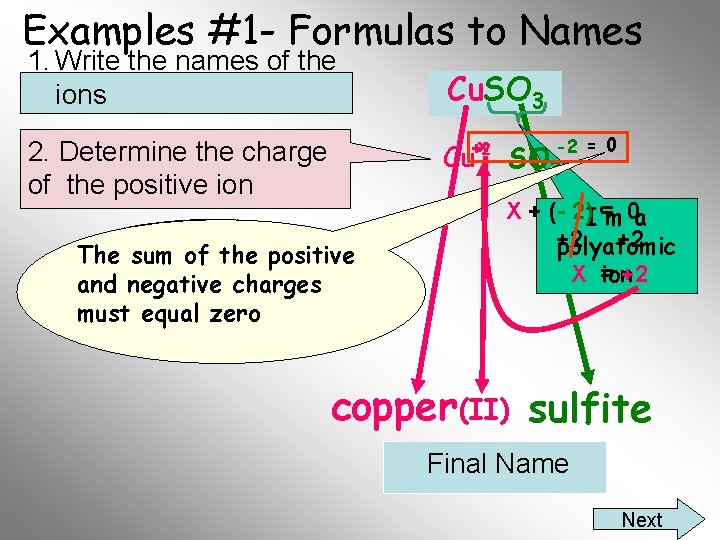
Examples #1 - Formulas to Names 1. Write the names of the ions Cu. SO 3 x +2 2. Determine the charge of the positive ion Cu The the positive You sum mustofknow the and negative charges charge on the sulfite must ion isequal -2 zero -2 = 0 SO 3 X + (- 2)I’m = 0 a +2 +2 polyatomic X = +2 ion copper (II) sulfite Final Name Next

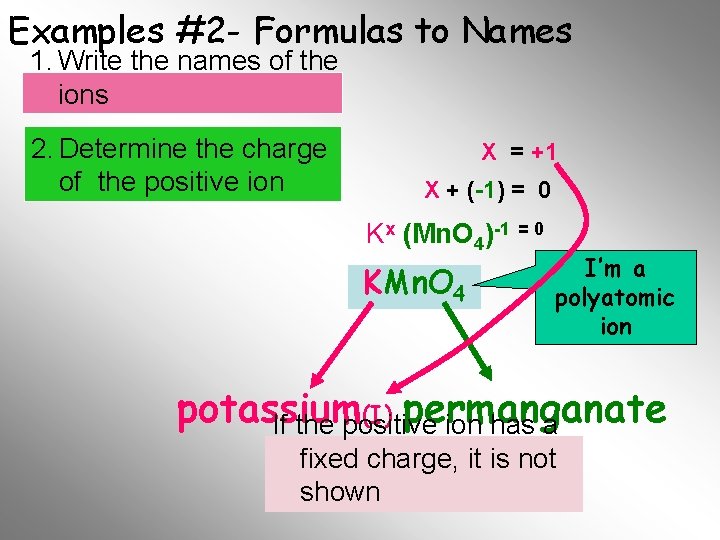
Examples #2 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +1 X + (-1) = 0 Kx (Mn. O 4)-1 = 0 KMn. O 4 I’m a polyatomic ion potassium (I) permanganate If the positive ion has a fixed. Final charge, it is not Name shown

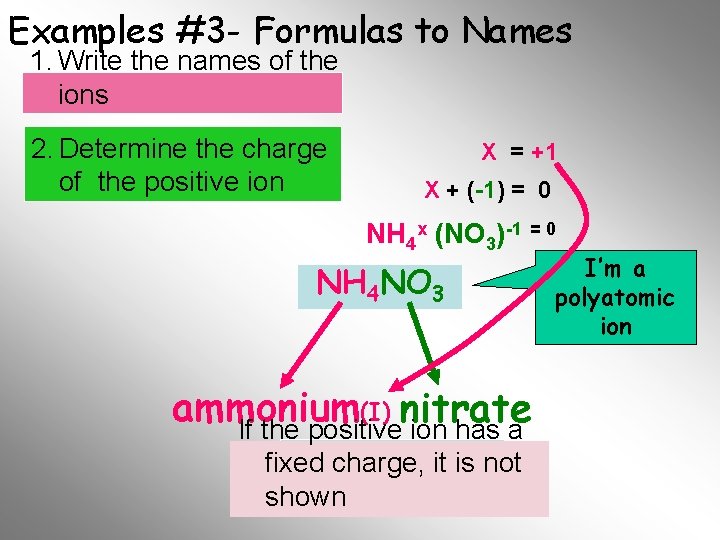
Examples #3 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +1 X + (-1) = 0 NH 4 x (NO 3)-1 = 0 NH 4 NO 3 (I) nitrate ammonium If the positive ion has a Name fixed. Final charge, it is not shown I’m a polyatomic ion

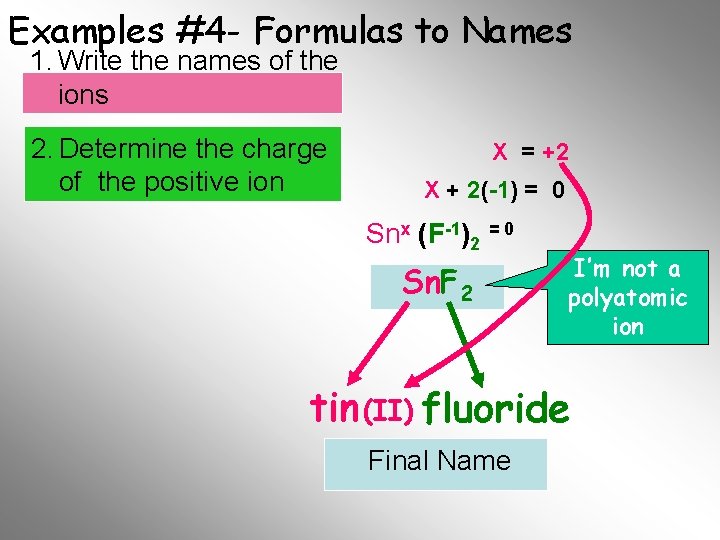
Examples #4 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +2 X + 2(-1) = 0 Snx (F-1)2 = 0 Sn. F 2 I’m not a polyatomic ion tin (II) fluoride Final Name

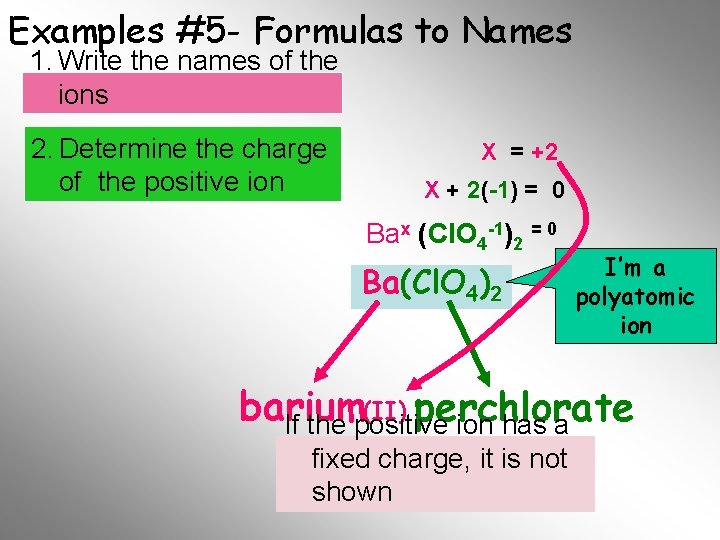
Examples #5 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +2 X + 2(-1) = 0 Bax (Cl. O 4 -1)2 = 0 Ba(Cl. O 4)2 I’m a polyatomic ion (II) perchlorate barium If the positive ion has a fixed. Final charge, it is not Name shown

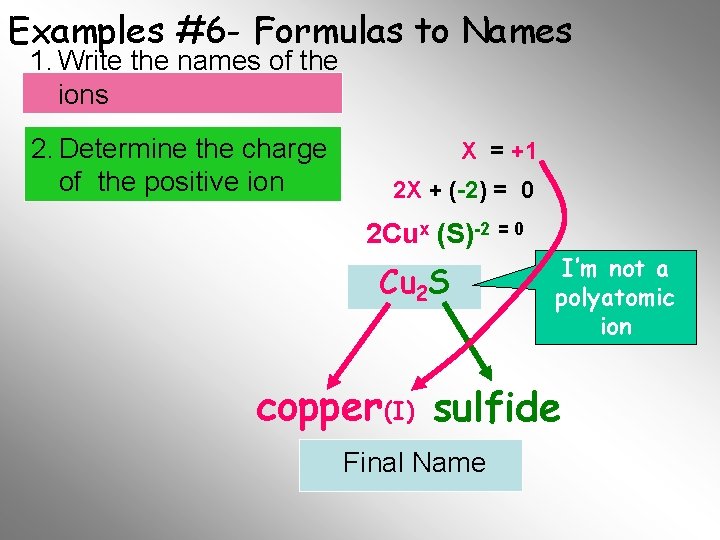
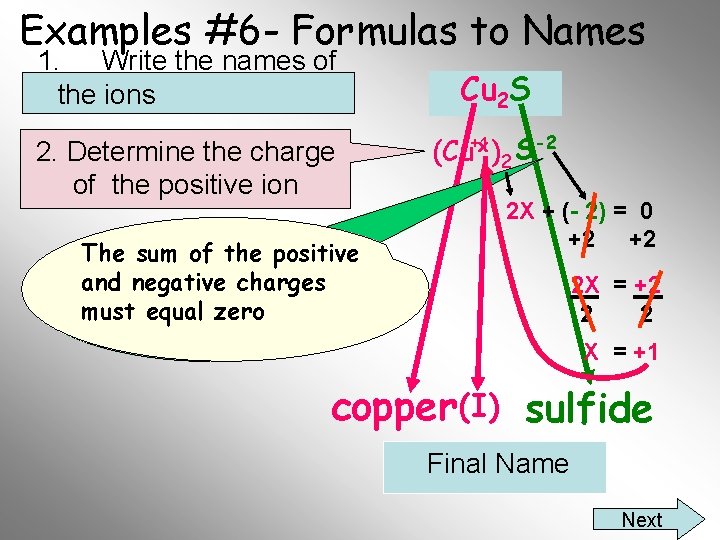
Examples #6 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +1 2 X + (-2) = 0 2 Cux (S)-2 = 0 Cu 2 S I’m not a polyatomic ion copper (I) sulfide Final Name

Examples #6 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion The the positive You sum mustofknow and negative charges charge on the sulfide must ion isequal -2 zero Cu 2 S (Cu+1 x )2 S -2 2 X + (- 2) = 0 +2 +2 2 X = +2 2 2 X = +1 copper (I) sulfide Final Name Next

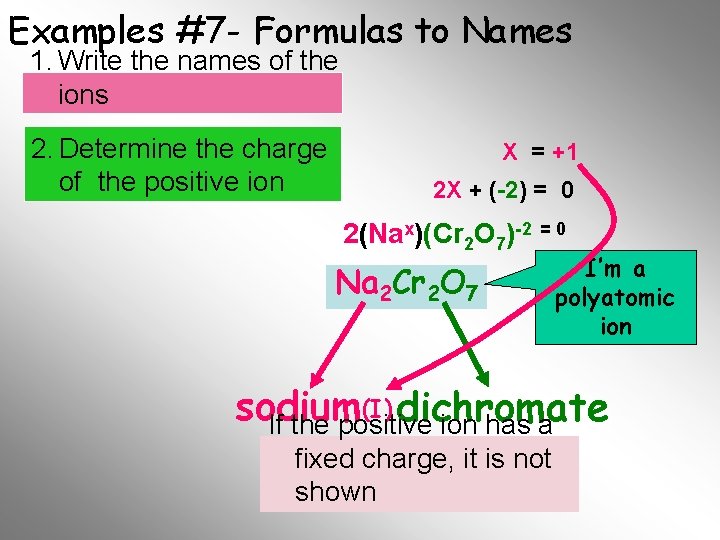
Examples #7 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +1 2 X + (-2) = 0 2(Nax)(Cr 2 O 7)-2 = 0 Na 2 Cr 2 O 7 I’m a polyatomic ion (I) dichromate sodium If the positive ion has a fixed. Final charge, it is not Name shown

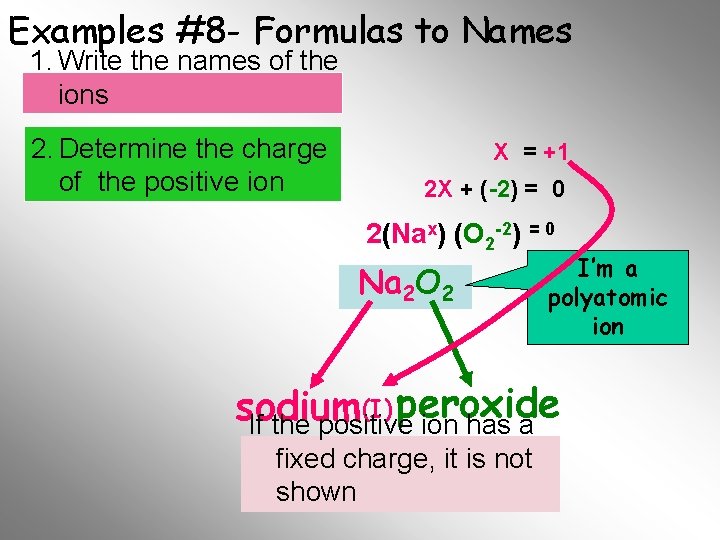
Examples #8 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +1 2 X + (-2) = 0 2(Nax) (O 2 -2) = 0 Na 2 O 2 I’m a polyatomic ion (I) peroxide sodium If the positive ion has a fixed. Final charge, it is not Name shown

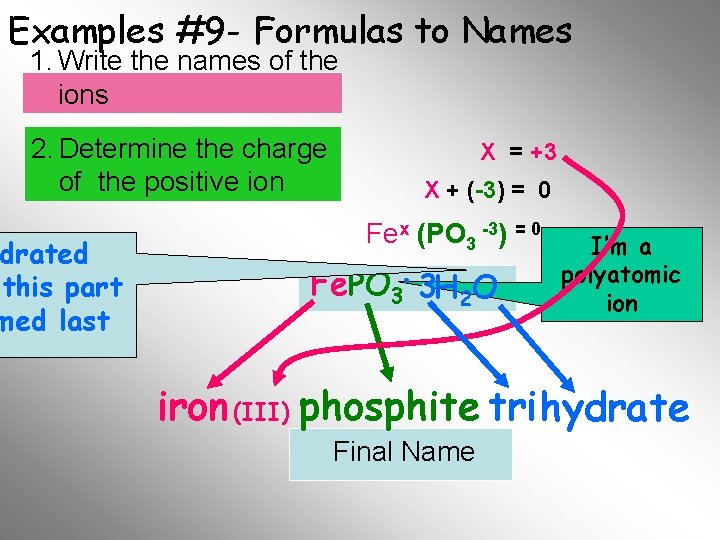
Examples #9 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion drated this part med last X = +3 X + (-3) = 0 Fex (PO 3 -3) = 0 Fe. PO 3. . 3 H O 33 H 22 O I’m a polyatomic ion iron (III) phosphite tri hydrate Final Name

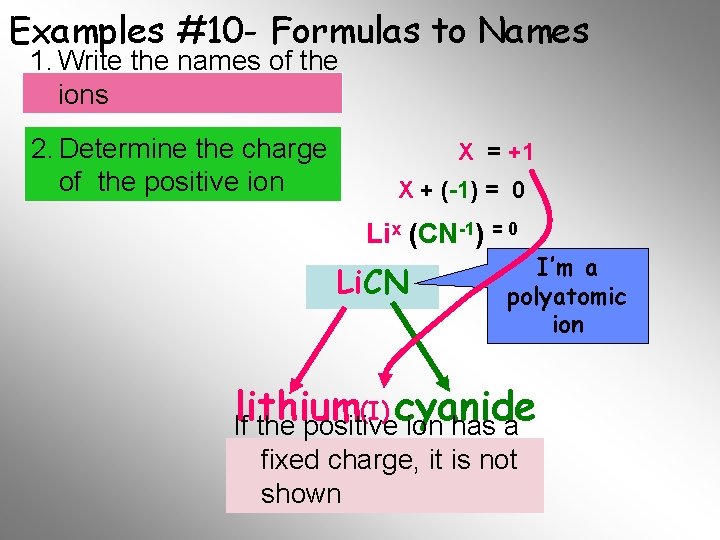
Examples #10 - Formulas to Names 1. Write the names of the ions 2. Determine the charge of the positive ion X = +1 X + (-1) = 0 Lix (CN-1) = 0 Li. CN I’m a polyatomic ion (I) cyanide lithium If the positive ion has a fixed Final charge, it is not Name shown

Writing Ionic Formulas • It is easier to write the formula of an ionic compound from its name than the reverse. The oxidation state (or charge) of all compounds is zero. This is the first naming system that requires the balancing of the positive and negative charges such that the result is zero. • The oxidation number of the negative ion must be memorized in all cases. Refer to the table of polyatomic ions.

• The positive ion is either one of those that has a fixed oxidation number or a “Roman Numeral” will follow the positive ion. Metals that have only one oxidation number must be memorized. These include metals in group 1 A, 2 A and the specific metals: Al, Zn, Cd & Ag. If there is a “Roman Numeral” is in the name, it represents the charge of one of the positive ions.

• Remember the total positive charge must equal to the total negative charge. The result is a compound which has no charge. Multiply the oxidation numbers of both the positive and negative ions by a number that will result in the smallest identical numbers of positive and negative charges. These multipliers represent the number of atoms of each ion required to give a neutral ionic compound.

• The formulas of hydrated compounds are written using a combination of both the stock system and prefixes. A prefix is used to denote the number of water molecules attached to the ionic formula. “hydrate” is used as the name indicating that water is attached. For example, copper(II) sulfate pentahydrate has the formula Cu. SO 4. 5 H 2 O (notice a dot separates the ionic compound from the water of hydration)

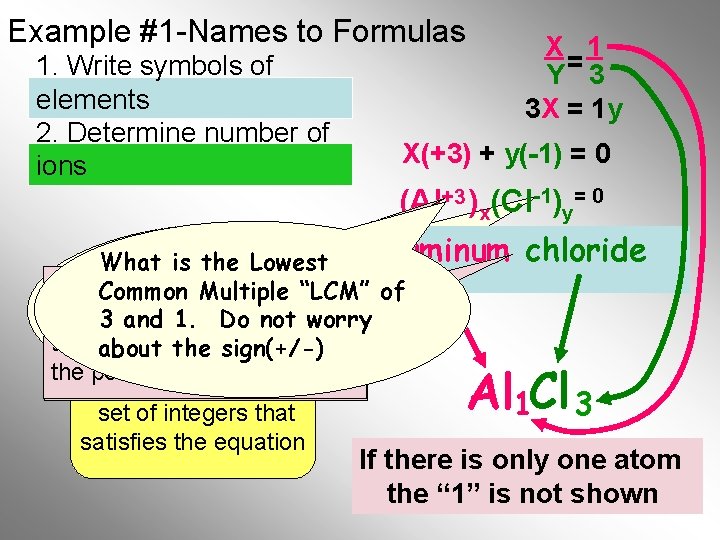
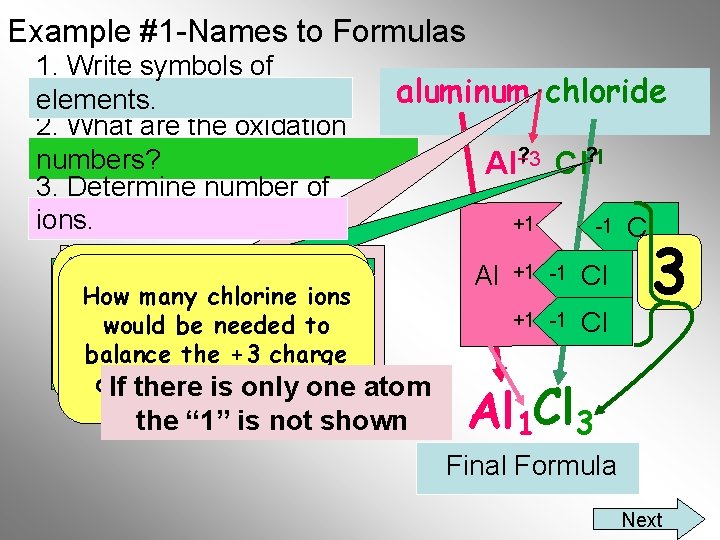
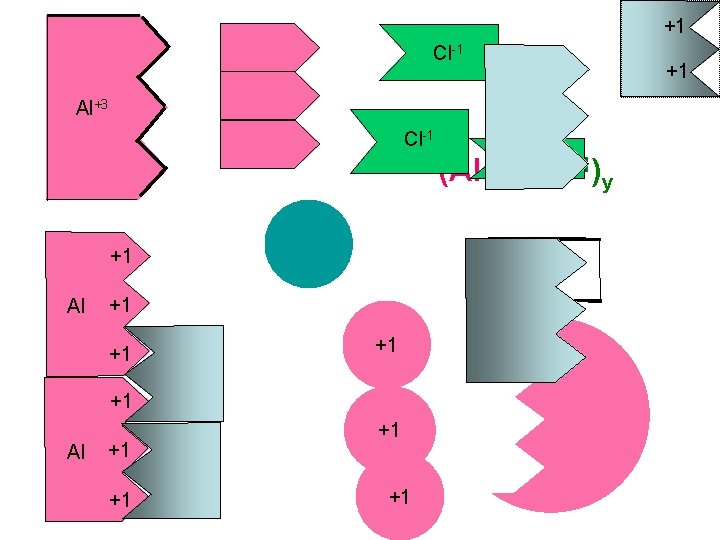
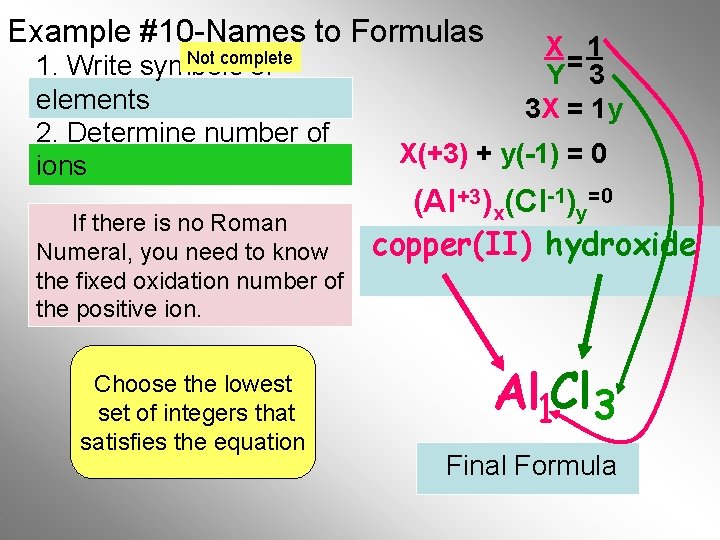
Example #1 -Names to Formulas 1. Write symbols of elements 2. Determine number of ions X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Al+3 )x(Cl-1)y= 0 aluminum chloride What is the Lowest If there is no Roman Common Multiple “LCM” of Numeral, to know 3 andyou 1. need Do not worry the fixed oxidation number of about the sign(+/-) the positive ion. Choose the lowest 1 3 set of integers that satisfies the equation Al Cl If there. Final is only one atom Formula the “ 1” is not shown

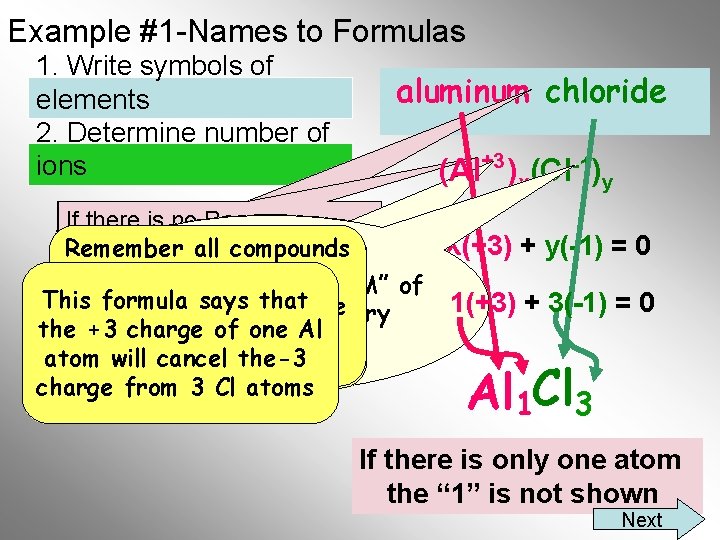
Example #1 -Names to Formulas 1. Write symbols of elements 2. Determine number of ions aluminum chloride (Al+3 )x(Cl-1)y If there is no Roman X(+3) + y(-1) = 0 Remember allmust compounds Numeral, you know the For aluminum, What is the Lowest For chloride, are neutral, thus oxidation number of the “LCM” of a 1 is multiplied Common Multiple a 3 is multiplied This formula says that 1(+3) + 3(-1) = 0 the total positive charge positive ion. times the +3 to give 3 and 1. Do not worry the -1 give the times +3 must charge of to one Al equal the a +3 charge about the sign(+/-) -3 charge atom willa cancel the-3 total negative charge from 3 Cl atoms Al 1 Cl 3 If there. Final is only one atom Formula the “ 1” is not shown Next

Example #1 -Names to Formulas 1. Write symbols of elements. 2. What are the oxidation numbers? 3. Determine number of ions. aluminum chloride If there is noall Roman Remember compounds You must also remember Numeral, youchlorine mustthus know the How aremany neutral, that chlorine is found in ions oxidation number ofcharge the would be needed to the total positive group 7 A, which always has positive ion. equal+3 the balance must a minus onethe charge on. If the aluminum ion? total negative charge there is only one atom the “ 1” is not shown ? Al+3 Cl? -1 +1 Al -1 +1 -1 Cl Cl 3 Al 1 Cl 3 Final Formula Next

+1 Cl-1 +1 Al+3 Cl-1 -1 (Al )x(Cl )y +1 Al +1 +1

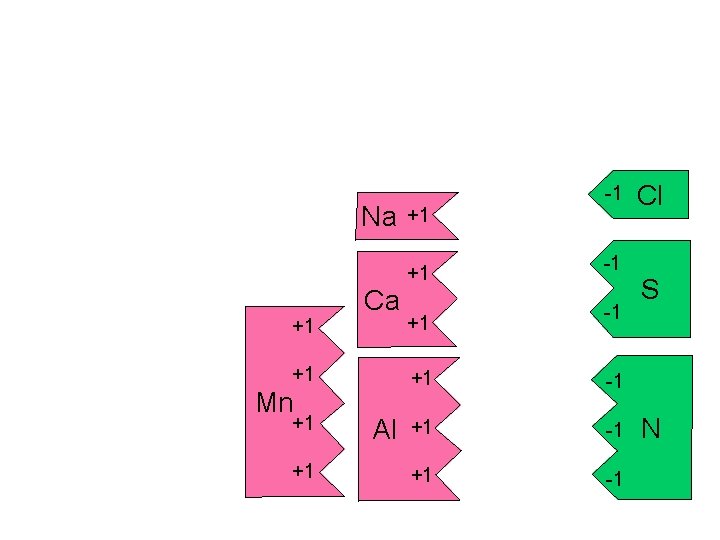
Na +1 Ca +1 Mn +1 +1 Al +1 +1 +1 -1 -1 -1 +1 -1 Cl S N

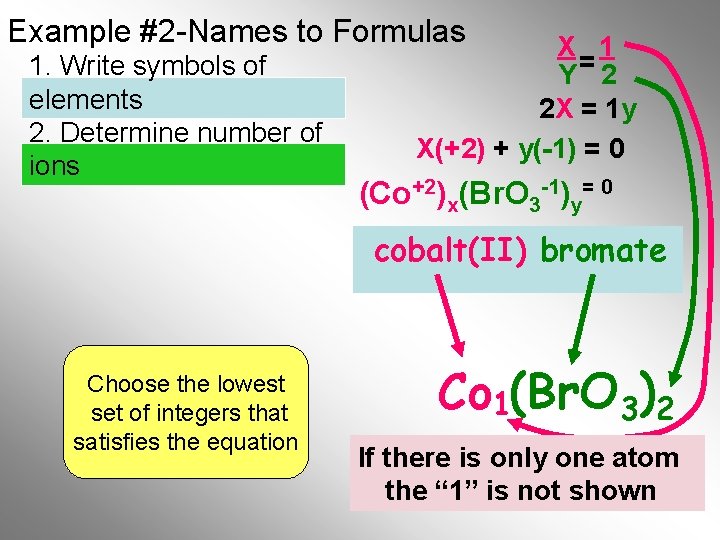
Example #2 -Names to Formulas 1. Write symbols of elements 2. Determine number of ions X 1 Y= 2 2 X = 1 y X(+2) + y(-1) = 0 (Co+2)x(Br. O 3 -1)y= 0 cobalt(II) bromate Choose the lowest set of integers that satisfies the equation Br. O 3)2 Co 1(Br. O If there. Final is only one atom Formula the “ 1” is not shown

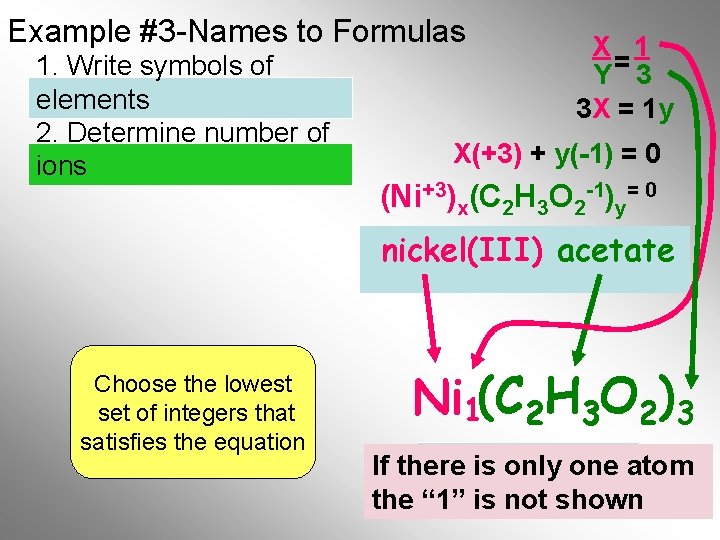
Example #3 -Names to Formulas 1. Write symbols of elements 2. Determine number of ions X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Ni+3)x(C 2 H 3 O 2 -1)y= 0 nickel(III) acetate Choose the lowest set of integers that satisfies the equation C 2 H 3 O 2)3 Ni 1(C Final Formula If there is only one atom the “ 1” is not shown

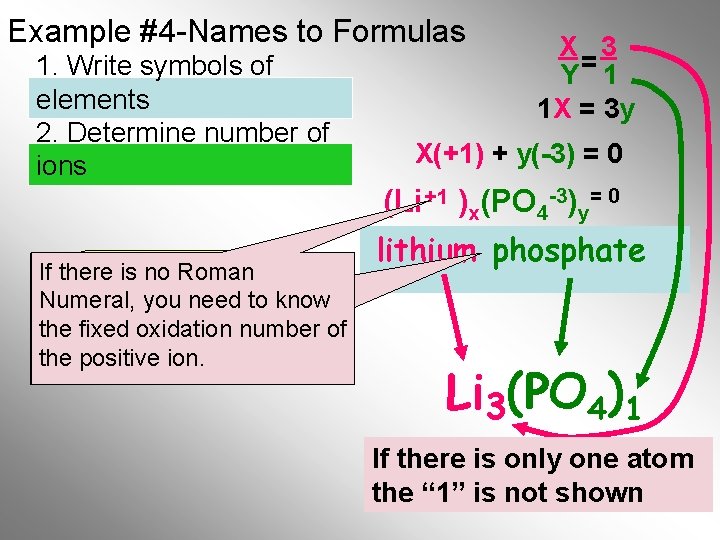
Example #4 -Names to Formulas 1. Write symbols of elements 2. Determine number of ions X 3 Y= 1 1 X = 3 y X(+1) + y(-3) = 0 (Li +1 )x(PO 4 -3)y= 0 If there is no Roman Choose lowest Numeral, youthe need to know set of integersnumber that of the fixed oxidation the equation the satisfies positive ion. lithium phosphate PO 4)1 Li 3(PO If there is only one atom Final Formula the “ 1” is not shown

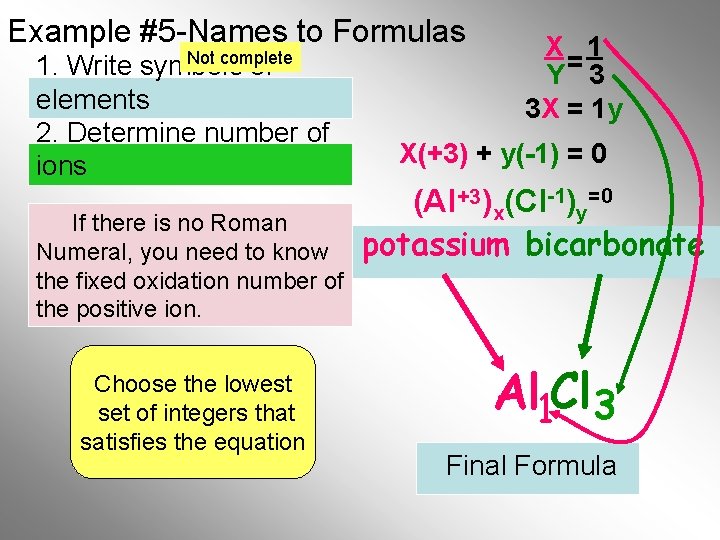
Example #5 -Names to Formulas Not complete 1. Write symbols of elements 2. Determine number of ions If there is no Roman Numeral, you need to know the fixed oxidation number of the positive ion. Choose the lowest set of integers that satisfies the equation X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Al +3)x(Cl-1)y=0 potassium bicarbonate Al 1 Cl 3 Final Formula

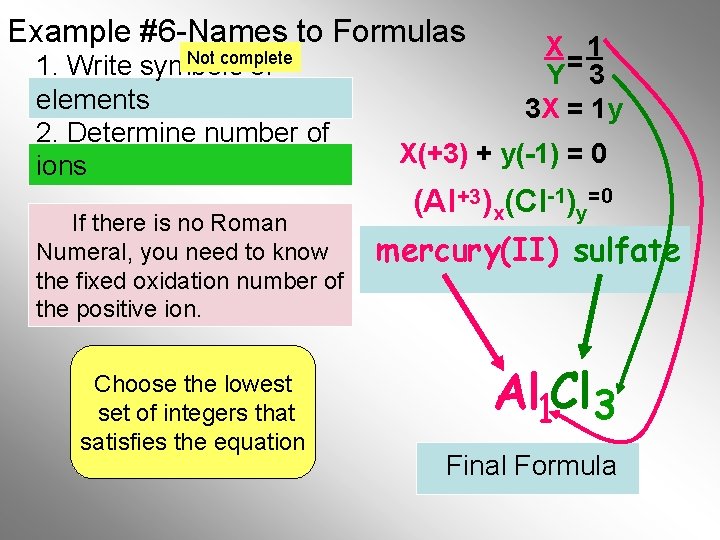
Example #6 -Names to Formulas Not complete 1. Write symbols of elements 2. Determine number of ions If there is no Roman Numeral, you need to know the fixed oxidation number of the positive ion. Choose the lowest set of integers that satisfies the equation X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Al +3)x(Cl-1)y=0 mercury(II) sulfate Al 1 Cl 3 Final Formula

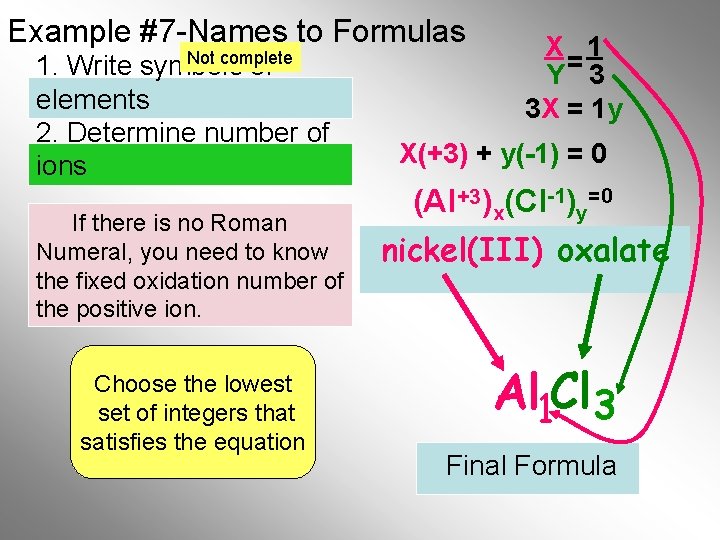
Example #7 -Names to Formulas Not complete 1. Write symbols of elements 2. Determine number of ions If there is no Roman Numeral, you need to know the fixed oxidation number of the positive ion. Choose the lowest set of integers that satisfies the equation X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Al +3)x(Cl-1)y=0 nickel(III) oxalate Al 1 Cl 3 Final Formula

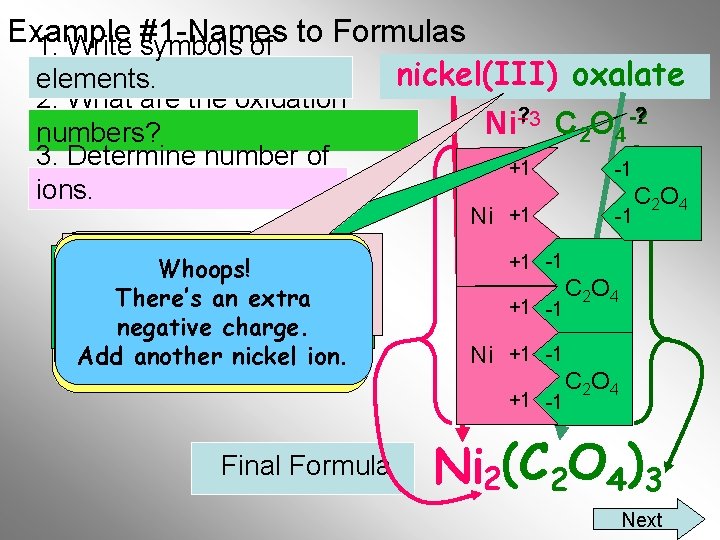
Example #1 -Names 1. Write symbols of to Formulas nickel(III) oxalate elements. 2. What are the oxidation numbers? 3. Determine number of ions. The Roman Numeral tells Remember all compounds How many oxalate ions You must remember that Whoops! youwould the positive ion has a are neutral, thus be needed to oxalate has a minus two There’s an extra charge of +3. the total positive charge balance the charge. +3 charge. negative must the on theequal nickel ion? ion. Add another nickel total negative charge ? Ni+3 C 2 O 4 -2? +1 Ni +1 -1 C 2 O 4 +1 -1 Ni +1 -1 Final Formula -1 C 2 O 4)3 Ni 2(C Next

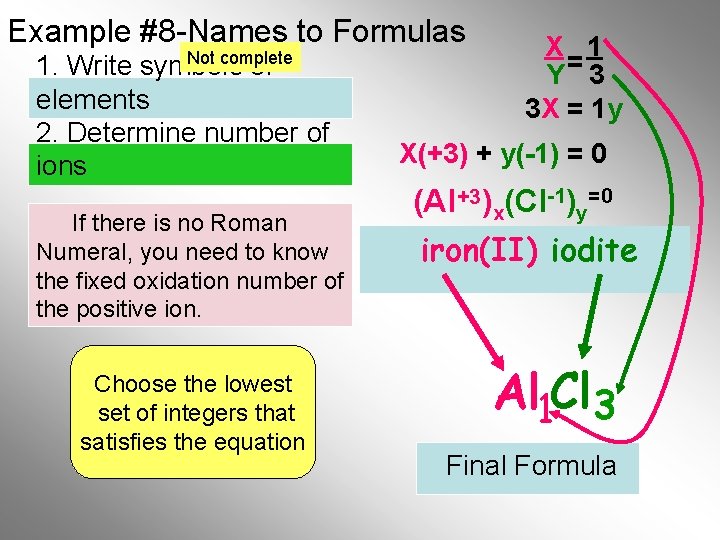
Example #8 -Names to Formulas Not complete 1. Write symbols of elements 2. Determine number of ions If there is no Roman Numeral, you need to know the fixed oxidation number of the positive ion. Choose the lowest set of integers that satisfies the equation X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Al +3)x(Cl-1)y=0 iron(II) iodite Al 1 Cl 3 Final Formula

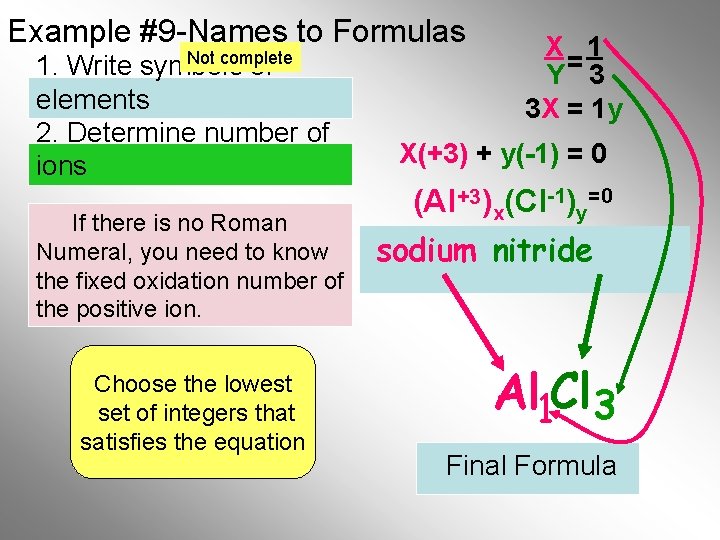
Example #9 -Names to Formulas Not complete 1. Write symbols of elements 2. Determine number of ions If there is no Roman Numeral, you need to know the fixed oxidation number of the positive ion. Choose the lowest set of integers that satisfies the equation X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Al +3)x(Cl-1)y=0 sodium nitride Al 1 Cl 3 Final Formula

Example #10 -Names to Formulas Not complete 1. Write symbols of elements 2. Determine number of ions If there is no Roman Numeral, you need to know the fixed oxidation number of the positive ion. Choose the lowest set of integers that satisfies the equation X 1 Y= 3 3 X = 1 y X(+3) + y(-1) = 0 (Al +3)x(Cl-1)y=0 copper(II) hydroxide Al 1 Cl 3 Final Formula

Practice Problems By now you should have an idea of what is expected when naming covalent binary compounds using prefixes. In order to master this naming system you need to practice until you feel proficient in naming compounds using prefixes.

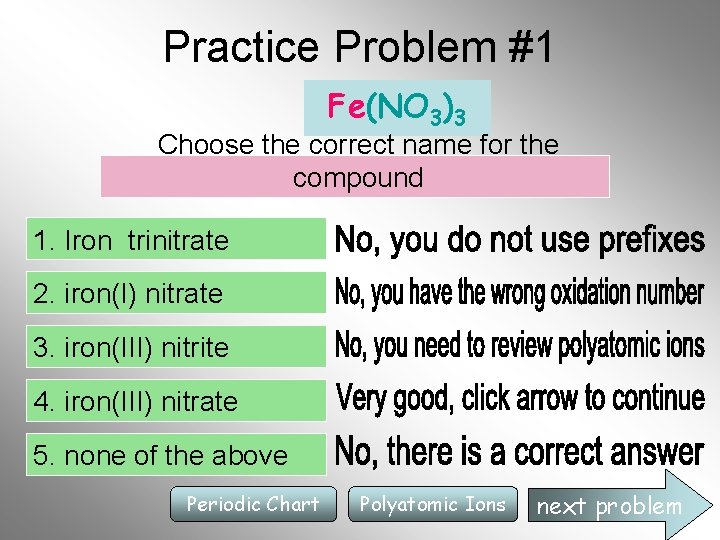
Practice Problem #1 Fe(NO 3)3 Choose the correct name for the compound 1. Iron trinitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #2 sodium chlorite Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

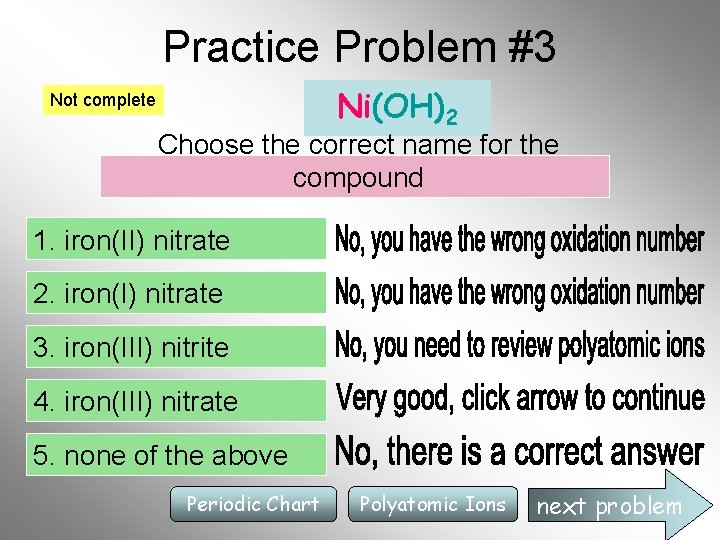
Practice Problem #3 Ni(OH)2 Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #4 Not complete manganese(II) sulfate Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #5 Ag 2 C 2 O 4 Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #6 Not complete sodium sulfate decahydrate Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #7 Na. H Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #8 Not complete cobalt(II) nitrate Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #9 Zn 3 N 2 Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #10 Not complete cadmium carbonate Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #11 Na. HSO 4 Choose the correct name for the compound 1. Iron trinitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #12 chromium(III) iodide Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #13 Sn(Cl. O 4)2 Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #14 Not complete hydrogen peroxide Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #15 Mn. O 2 Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #16 Not complete aluminum hypobromite Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #17 Co. Cl 2. 6 H 2 O Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

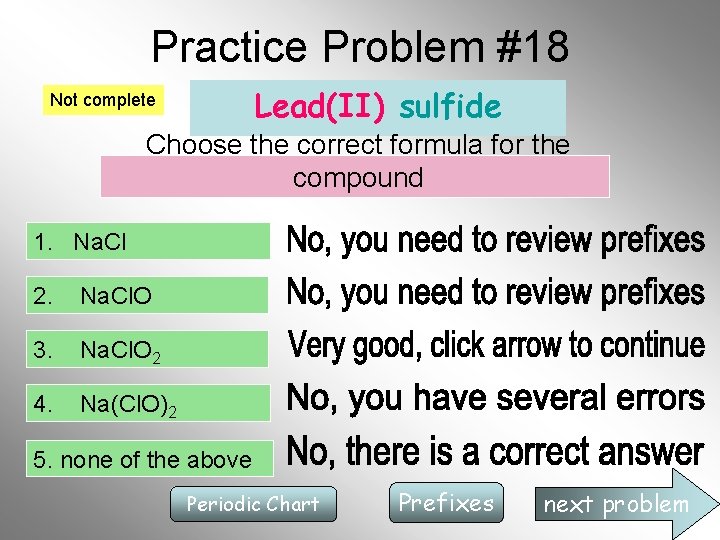
Practice Problem #18 Lead(II) sulfide Not complete Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

Practice Problem #19 Ag 3 As. O 4 Not complete Choose the correct name for the compound 1. iron(II) nitrate 2. iron(I) nitrate 3. iron(III) nitrite 4. iron(III) nitrate 5. none of the above Periodic Chart Polyatomic Ions next problem

Practice Problem #20 Not complete potassium thiocyanate Choose the correct formula for the compound 1. Na. Cl 2. Na. Cl. O 3. Na. Cl. O 2 4. Na(Cl. O)2 5. none of the above Periodic Chart Prefixes next problem

POLYATOMIC IONS Ions with -1 charge perbromate Br. O 4 -1 bromate Br. O 3 -1 bromite Br. O 2 -1 hypobromite Br. O-1 perchlorate Cl. O 4 -1 chlorate Cl. O 3 -1 chlorite Cl. O 2 -1 hypochlorite Cl. O-1 periodate IO 4 -1 iodate IO 3 -1 iodite IO 2 -1 hypoiodite IO-1 nitrate NO 3 -1 nitrite NO 2 -1 hydroxide OH-1 cyanide CN-1 thiocyanate SCN-1 acetate C 2 H 3 O 2 -1 Permanganate Mn. O 4 -1 bicarbonate HCO 3 -1 Ions with a -2 Charge carbonate CO 3 -2 phthalate C 8 H 4 O 4 -2 sulfate SO 4 -2 sulfite chromate dichromate oxalate peroxide SO 3 -2 Cr. O 4 -2 Cr 2 O 7 -2 C 2 O 4 -2 O 2 -2 Ions with a -3 Charge phosphate PO 4 -3 phosphite PO 3 -3 arsenate As. O 4 -3 Ions with +1 charge ammonium ion NH 4+1 return

Oxidation Numbers (most common) -2 -3 -4 +3 +2 +1 -1 0 1 A H 2 A Li +1 +2 Be Click on element to see its oxidation number(s) Na Mg K Ca Sc Ti Rb Sr +2 +3 Y V +2 +4 7 A 3 A 4 A 5 A 6 A 8 A H He B C N O F Ne Al Si P S Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Ce Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi I Xe Po At Rn Fr Ra Ac Rf Db Sg Rh Hs Mt return Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr