Ionic Compound Nomenclature Vocabulary polyatomic ion multivalent Olympic

Ionic Compound Nomenclature Vocabulary • polyatomic ion • multivalent “Olympic Pieces”, Banksy, British, 2012

Why do we need names for people?

Why do we need names for compounds?

So how do we name ionic compounds? It’s easy! Name each of the ions in the compound. There’s just a couple of things to keep in mind.

Rule #1 Name the cation first and the anion second (just like they are in the formula).

Rule #2 If the anion is an element, change the ending of the element to “-ide. ” Examples: chlorine becomes chloride phosphorous becomes phosphide

Rule #3 If the anion is a polyatomic ion, do not change its name. (The polyatomic ions are listed on your polyatomic ion sheet. If you have lost yours, you can download a new one on my website. )
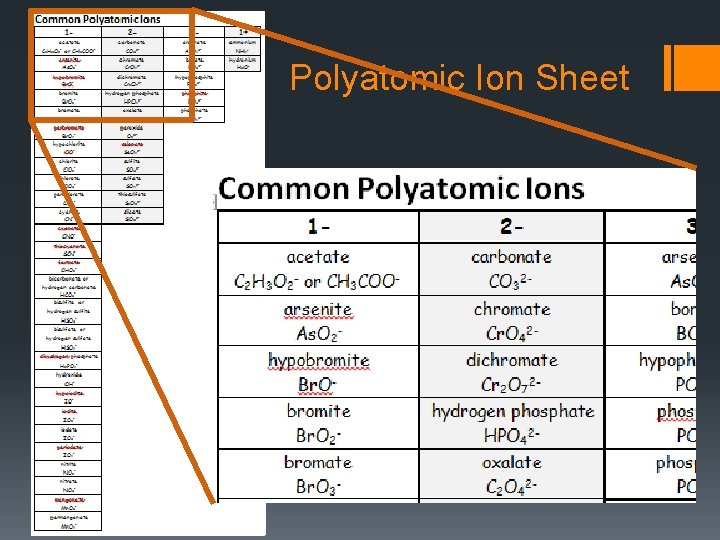
Polyatomic Ion Sheet

Rule #4 If the cation can have more than one charge (if it is multivalent), use a Roman numeral to indicate the charge of the cation. (These are generally the transition metals and tin and lead. There are some exceptions we’ll look at later. )
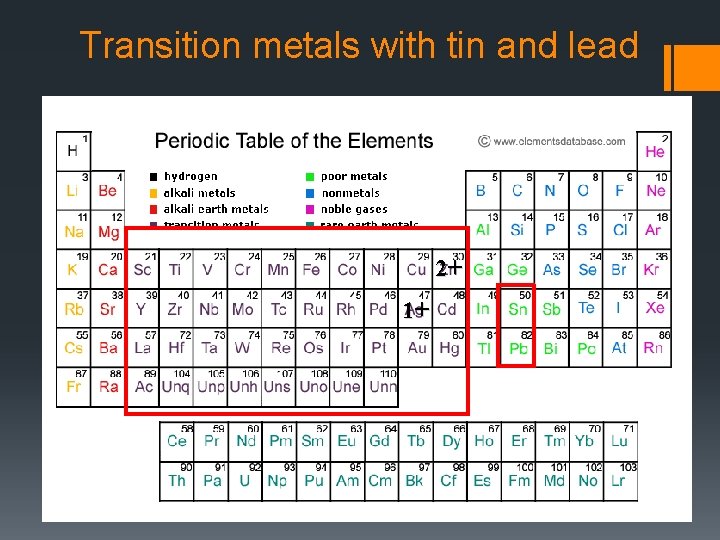
Transition metals with tin and lead 2+ 1+

Practice #1 §Name the following ionic compound: Na. Cl §Rule #1: sodium chlorine §Rule #2: sodium chloride §Rule #3: N/A §Rule #4: N/A §So the compound is sodium chloride

Practice #2 §Name the following ionic compound: Al 2 O 3 §Rule #1: aluminum oxygen §Rule #2: aluminum oxide §Rule #3: N/A §Rule #4: N/A §So the compound is aluminum oxide

Practice #3 §Name the following ionic compound: Na 2 SO 4 §Rule #1: sodium sulfate §Rule #2: N/A §Rule #3: sodium sulfate §Rule #4: N/A §So the compound is sodium sulfate

Practice #4 §Name the following ionic compound: Ca 3(PO 3)2 §Rule #1: calcium phosphite §Rule #2: N/A §Rule #3: calcium phosphite §Rule #4: N/A §So the compound is calcium phosphite

Practice #5 §Name the following ionic compound: Fe. Cl 2 §Rule #1: iron chlorine §Rule #2: iron chloride §Rule #3: N/A §Rule #4: this one applies this time because iron is a transition metal and can have multiple charges (it’s multivalent), so let’s take a closer look…

Multivalency §Most transition metals (and lead and tin) are multivalent (i. e. , they can have more than one charge. ) This is in contrast to the elements in groups 1, 2, and 13 which can only have one charge. Iron is a transition metal, so in order to correctly name this compound, we need to figure out what charge is on the iron cation.
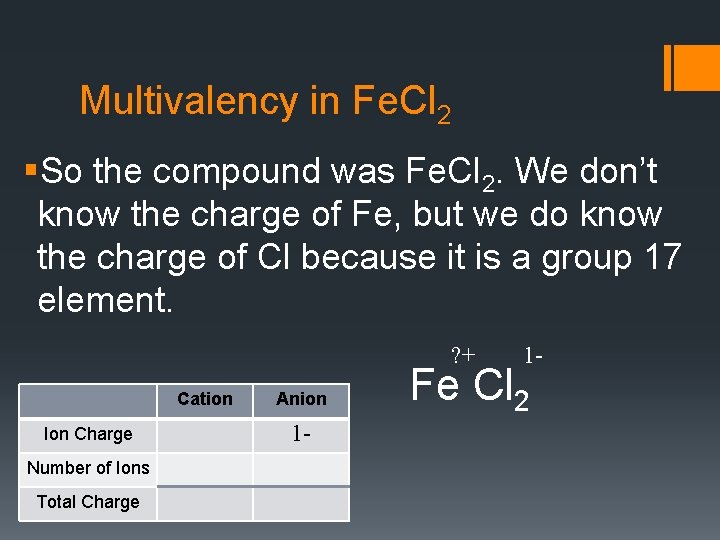
Multivalency in Fe. Cl 2 §So the compound was Fe. Cl 2. We don’t know the charge of Fe, but we do know the charge of Cl because it is a group 17 element. ? + Cation Ion Charge Number of Ions Total Charge Anion 1 - 1 - Fe Cl 2
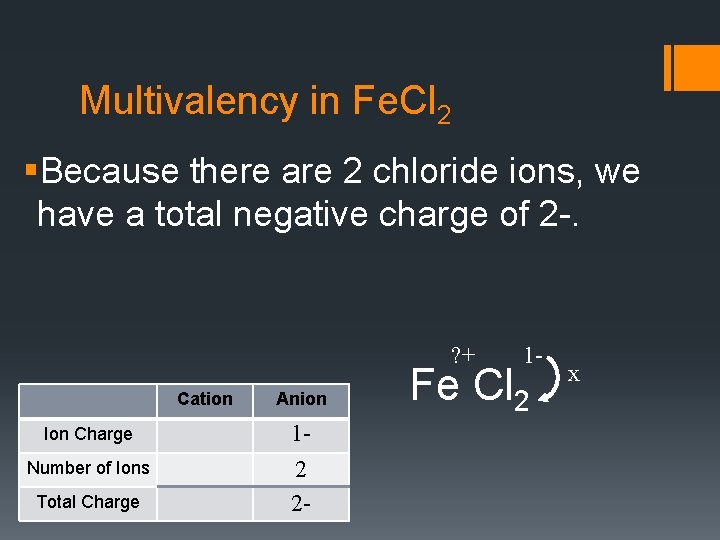
Multivalency in Fe. Cl 2 §Because there are 2 chloride ions, we have a total negative charge of 2 -. ? + Cation Ion Charge Number of Ions Total Charge Anion 12 2 - 1 - Fe Cl 2 x
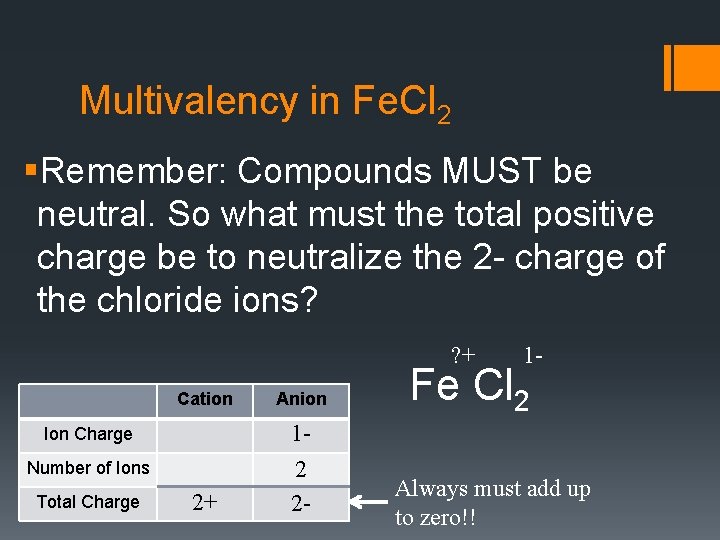
Multivalency in Fe. Cl 2 §Remember: Compounds MUST be neutral. So what must the total positive charge be to neutralize the 2 - charge of the chloride ions? ? + Cation Anion 2+ 12 2 - Ion Charge Number of Ions Total Charge 1 - Fe Cl 2 Always must add up to zero!!
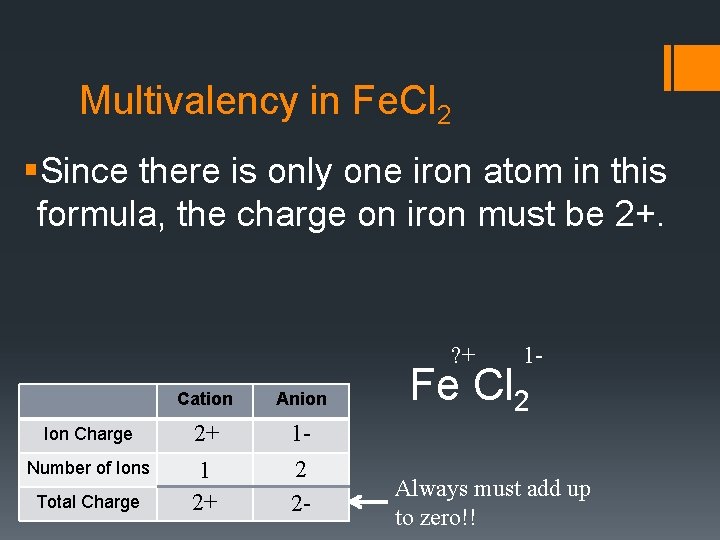
Multivalency in Fe. Cl 2 §Since there is only one iron atom in this formula, the charge on iron must be 2+. ? + Cation Anion Ion Charge 2+ Number of Ions 1 2+ 12 2 - Total Charge 1 - Fe Cl 2 Always must add up to zero!!

Multivalency in Fe. Cl 2 §So in the name, we will use a Roman numeral 2 to indicate the charge of iron. 2+ 1 - Fe Cl 2 §It is iron(II) chloride

Practice #5 §Name the following ionic compound: Fe. Cl 2 §Rule #1: iron chlorine §Rule #2: iron chloride §Rule #3: N/A §Rule #4: iron(II) chloride §So the name is iron(II) chloride

Practice #6 §Name the following ionic compound: Cr. F 3 §Rule #1: chromium fluorine §Rule #2: chromium fluoride §Rule #3: N/A §Rule #4: chromium(III) fluoride §So the name is chromium(III) fluoride

Practice #7 §Name the following ionic compound: Cu 3 P §Rule #1: copper phosphorous §Rule #2: copper phosphide §Rule #3: N/A §Rule #4: let’s look at this one more closely

Practice #7 (Cu 3 P) §Copper is a transition metal so we need to determine its charge in this compound. We don’t know copper’s charge, but we do know phosphorous’ charge because it is in group 15. ? + Cation Ion Charge Number of Ions Total Charge Anion 31 3 - Cu 3 P 3 - x

Practice #7 (Cu 3 P) §You might think that copper will be 3+ to cancel out the 3 - charge. But no. Remember there are 3 ions of copper in this compound that add up to 3+. ? + Cation Anion 3 3+ 31 3 - Ion Charge Number of Ions Total Charge Cu 3 P 3 -

Practice #7 (Cu 3 P) §So what is the charge on each copper ion? § 1+ §So we will name this copper(I) phosphide ? + Ion Charge Number of Ions Total Charge Cation Anion 1+ 3 3+ 31 3 - Cu 3 P 3 -

Practice #8 §Name the following ionic compound: Ni 2(C 2 O 4)3 §Rule #1: nickel oxalate §Rule #2: N/A §Rule #3: nickel oxalate §Rule #4: nickel is a transition metal, so let’s look at this more closely

Practice #8 We don’t know nickel’s charge, but we do know oxalate’s charge. By using oxalate’s charge we can determine nickel’s charge. ? + 2 - Ni 2(C 2 O 4)3

Practice #8 §First we need to figure out the total negative charge. §What is the charge on oxalate (the anion)? §How many oxalate ions are there? §So what is the total negative charge? ? + Cation Ion Charge Number of Ions Total Charge Anion 23 6 - 2 - Ni 2(C 2 O 4)3 x

Practice #8 §Now let’s figure out information about the cation, nickel. §Because the compound must be neutral, what is the total positive charge in the compound? §How many nickel ions are there? §So what is the charge on each nickel ion? ? + Cation Anion 2 6+ 23 6 - Ion Charge Number of Ions Total Charge 2 - Ni 2(C 2 O 4)3 Always must add up to zero!!

Practice #8 §Now let’s figure out information about the cation, nickel. §Because the compound must be neutral, what is the total positive charge in the compound? §How many nickel ions are there? §So what is the charge on each nickel ion? 3+ Ion Charge Number of Ions Total Charge Cation Anion 3+ 2 6+ 23 6 - 2 - Ni 2(C 2 O 4)3 Always must add up to zero!!

Practice #8 §So now that we know the charge on each nickel ion, we can name this compound. 3+ 2 - Ni 2(C 2 O 4)3 §It is nickel(III) oxalate.

Practice #9 §Name the following ionic compound: Ti. O 2 §Rule #1: titanium oxygen §Rule #2: titanium oxide §Rule #3: N/A §Rule #4: titanium is a transition metal, so let’s look at this more closely

Practice #9 ? + 2 - Ti O 2
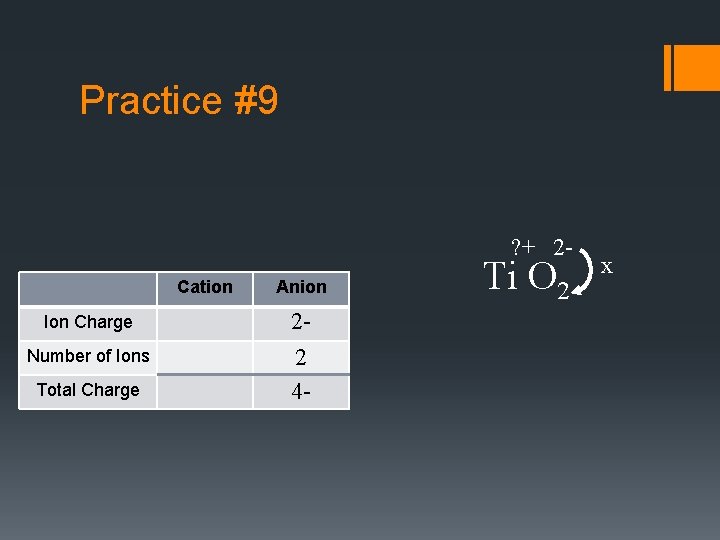
Practice #9 ? + 2 - Cation Ion Charge Number of Ions Total Charge Anion 22 4 - Ti O 2 x

Practice #9 ? + 2 - Cation Anion 1 4+ 22 4 - Ion Charge Number of Ions Total Charge Ti O 2 Always must add up to zero!!

Practice #9 4+ 2 - Cation Anion Ion Charge 4+ Number of Ions 1 4+ 22 4 - Total Charge Ti O 2 Always must add up to zero!!

Practice #9 §So now that we know the charge on each nickel ion, we can name this compound. 4+ 2 - Ti O 2 §It is titanium(IV) oxide
- Slides: 39