Ionic Compound Names and Formulas Monovalent Ionic Binary
Ionic Compound Names and Formulas
Monovalent Ionic Binary Compounds l l “+” means lose “-” means gain The number (+1, +2, +3)represents the number of electrons gained or lost to achieve a noble gas configuration Also called “oxidation number” Is at the top of the (family) column on the periodic table
Monovalent Ionic Binary Compounds l l Cation has only one possible charge. Group 1 A and 2 A metals have only one oxidation number [+1, +2] Groups 3 A, 4 A, 5 A, 6 A, and 7 A usually only have one oxidation number [+3, +4, -3, -2, -1] Monovalent Transition Metals: l l Zn = +2 Ag = +1 Cd = +2 Ni = +2

Naming Ionic Compounds l l l Name cation first and the anion second Monoatomic cations use element names Monoatomic anions take the name from the root of the element name plus the suffix “ide”

Polyatomic Ions l l Ions made up of more than one atom Act as an individual ion Never change the subscripts of the ion If a subscript is needed, put parentheses around the polyatomic ion

Polyatomic Ions l l l If a compound contains a polyatomic ion, use the name of the ion Some polyatomic anions have a names ending in -ite for the lower # of oxygens and –ate for more oxygens. NO 2 - nitrite NO 3 - nitrate
Divalent Ionic Binary Compounds l l l Cation can have two charges, so the charge must be named. Transitional metals and metals on the right side of the periodic table often have more than one oxidation number. The oxidation number will be written with a Roman numeral after the name of the metal. This is known as the stock system. (see handout)

Divalent Ionic Binary Compounds l l A Roman numeral in parentheses, preceded by the name of the element, is used for elements that can form more than one positive ion. This is usually seen with metals. Fe 2+ Iron (II) Cu+ Copper (I) Fe 3+ Iron (III) Cu 2+ Copper (II)
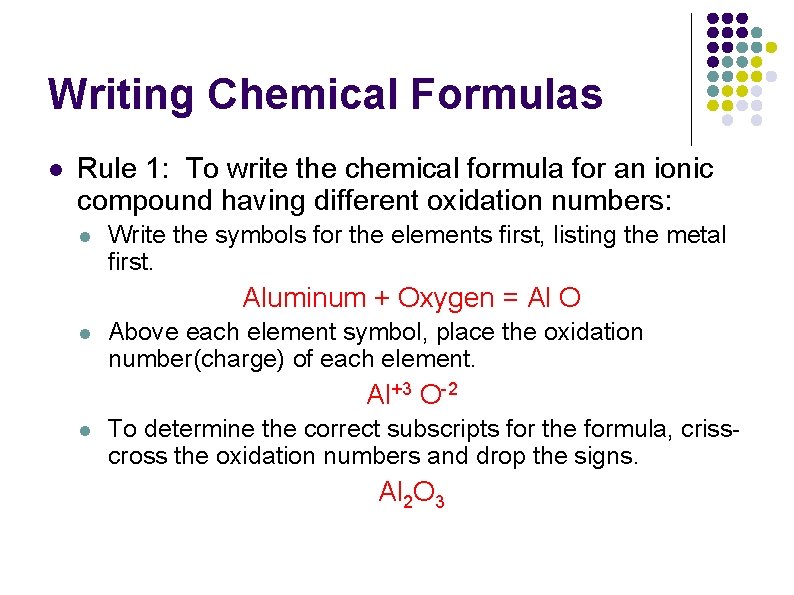
Writing Chemical Formulas l Rule 1: To write the chemical formula for an ionic compound having different oxidation numbers: l Write the symbols for the elements first, listing the metal first. Aluminum + Oxygen = Al O l Above each element symbol, place the oxidation number(charge) of each element. Al+3 O-2 l To determine the correct subscripts for the formula, crisscross the oxidation numbers and drop the signs. Al 2 O 3
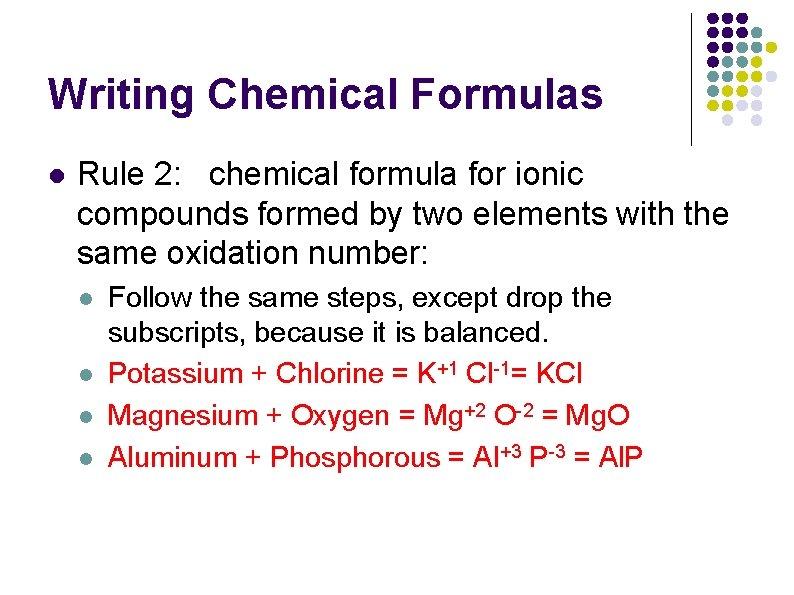
Writing Chemical Formulas l Rule 2: chemical formula for ionic compounds formed by two elements with the same oxidation number: l l Follow the same steps, except drop the subscripts, because it is balanced. Potassium + Chlorine = K+1 Cl-1= KCl Magnesium + Oxygen = Mg+2 O-2 = Mg. O Aluminum + Phosphorous = Al+3 P-3 = Al. P
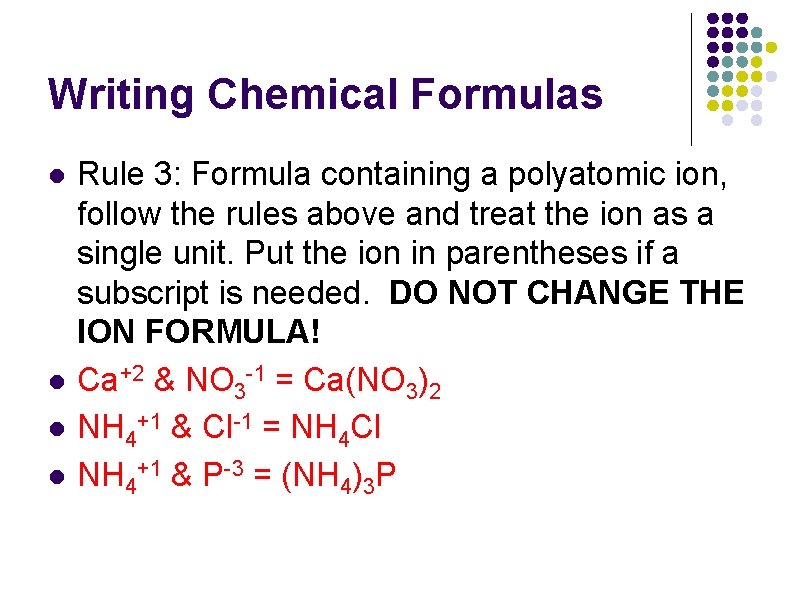
Writing Chemical Formulas l l Rule 3: Formula containing a polyatomic ion, follow the rules above and treat the ion as a single unit. Put the ion in parentheses if a subscript is needed. DO NOT CHANGE THE ION FORMULA! Ca+2 & NO 3 -1 = Ca(NO 3)2 NH 4+1 & Cl-1 = NH 4 Cl NH 4+1 & P-3 = (NH 4)3 P
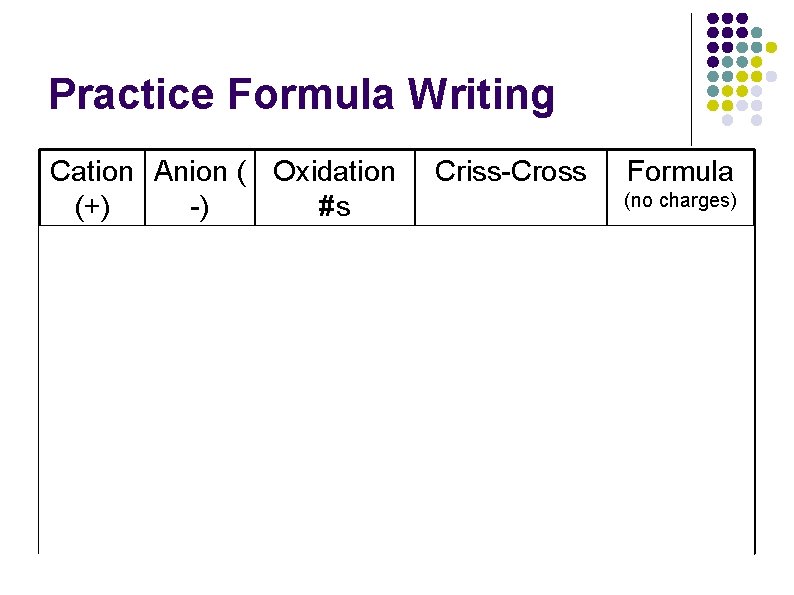
Practice Formula Writing Cation Anion ( Oxidation Criss-Cross Formula (no charges) (+) -) #s Na Cl Na+1 Cl-1 Na-1 Cl+1 Na. Cl H O H+1 O-2 H-2 O+1 H 2 O Ca Br Ca+2 Br-1 Ca-1 Br+2 Ca. Br 2 Li Cr 2 O 7 -2 Li+1 Cr 2 O 7 -2 Li-2(Cr 2 O 7)+1 Li 2 Cr 2 O 7 K I K+1 I-1 K-1 I+1 KI Ca PO 4 -3 Ca+2 PO 4 -3 Ca-3(PO 4)+2 Ca 3(PO 4)2

Naming Molecules l l Use your Naming Compounds handout and follow the steps for naming binary molecular compounds. Ready? ? ?

Naming Compounds l Rule 1: For an ionic compound that contains only two elements (binary compound), name the metal first, then add the suffix –ide to the end of the nonmetal l l Na. Cl = sodium chloride K 2 S = potassium sulfide Ca. O = calcium oxide Al 2 O 3 = aluminum oxide

Naming Compounds l Rule 2: For compounds containing three elements (ternary compounds), such as a metal combined with a polyatomic ion that behaves as a nonmetal, name the metal first and name the polyatomic ion last. l l l Na. NO 3 = sodium nitrate Na. NO 2 = sodium nitrite Ca. SO 4 = calcium sulfate KNOW YOUR POLYATOMIC IONS!!!!!

Naming Compounds l Rule 3: For compounds containing two polyatomic ions, one acting as the metal and the other as a nonmetal, name the polyatomic ion with the positive oxidation number first, and then the polyatomic ion with the negative oxidation number. l l l (NH 4)2 SO 4 = ammonium sulfate NH 4 OH = ammonium hydroxide Note: If ammonium is paired with a nonmetal, the nonmetal will end in –ide l (NH 4)3 N = ammonium nitride

Naming Compounds l Rule 4: If a compound contains a metal with an oxidation number that can vary, indicate the oxidation number of the metal with a Roman numeral written after the name of the metal. These metals include: Cr, Mn, Fe, Co, Ni, Cu, Hg, Sn, & Pb. l l l Fe. O = iron (II) oxide Fe 2 O 3 = iron (III) oxide You may also use the common names l l Fe. O = ferrous oxide Fe 2 O 3 = ferric oxide
Naming Compounds l Rule 5: For binary covalent compounds (containing all nonmetals), use prefixes to represent the atoms of an element. The name ends in –ide. Mono = 1 Hexa = 6 Di = 2 Hepta = 7 Tri = 3 Octa = 8 Tetra = 4 Nona = 9 Penta = 5 Deca = 10 l l CO = carbon monoxide CO 2 = carbon dioxide NO = nitrogen monoxide P 2 O 3 = diphosphorous trioxide

Binary Acids l l Contains hydrogen and another element Name begins with “hydro” and ends with “ic”. The rest of the name is made up of the root of the second element. l Hydro-”element”-ic acid

Oxyacids l l l Name consists of the root of the anion (usually a polyatomic ion), a suffix “ic” or “ous” and the word acid. “ate” polyatomic ion names are replaced with “ic” “ite” polyatomic ion names are replaced with “ous”
- Slides: 20