Ionic bondscrystalline structure Ionic bonds usually involve cations
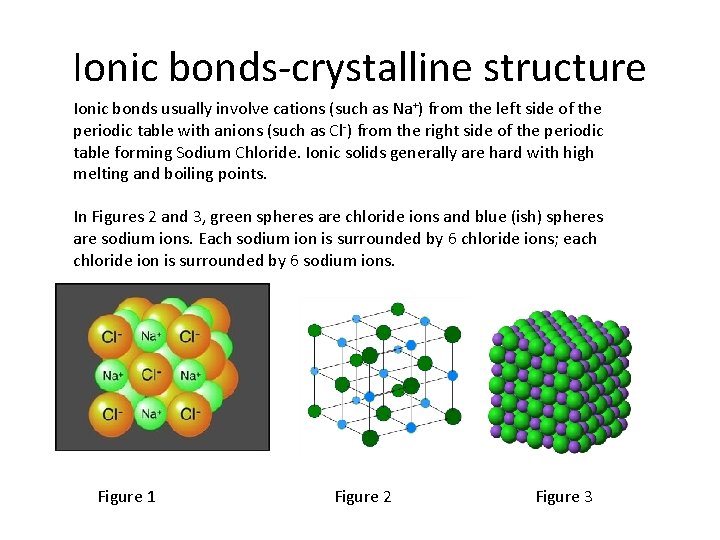
Ionic bonds-crystalline structure Ionic bonds usually involve cations (such as Na+) from the left side of the periodic table with anions (such as Cl-) from the right side of the periodic table forming Sodium Chloride. Ionic solids generally are hard with high melting and boiling points. In Figures 2 and 3, green spheres are chloride ions and blue (ish) spheres are sodium ions. Each sodium ion is surrounded by 6 chloride ions; each chloride ion is surrounded by 6 sodium ions. Figure 1 Figure 2 Figure 3
![Lewis dot structure (Covalent Bonds) 1. [ Google-youtube “ Drawing Lewis Dot Structures”] Draw Lewis dot structure (Covalent Bonds) 1. [ Google-youtube “ Drawing Lewis Dot Structures”] Draw](http://slidetodoc.com/presentation_image_h/c943949574938ce0f8314fa83b32b064/image-2.jpg)
Lewis dot structure (Covalent Bonds) 1. [ Google-youtube “ Drawing Lewis Dot Structures”] Draw the orbital pattern diagram, if necessary, to determine the number of valance ( last s and p) electrons. 2. Elements in the same chemical family have the same number (and arrangement) of valence electrons (example: Halogens such as F and Cl). 3. Place 4 electrons (dots) first singly around the atomic symbol (Li to C). Then place the other electrons in pairs (N to Ne).
Lewis dot structure (Covalent Bonds) Draw the Lewis dot structures for the elements, compounds, or ions below: A. Count the total number of valence electrons for each element. + Ions such as NH 4+ have fewer (8 not 9) electrons to be considered. - Ions such as SO 42 - have more (32 not 30) electrons to be considered. B. Arrange the electrons in the structure so that there are 2 e- ’s around each hydrogen (Duet rule) and 8 e- ’s around the other elements (Octet rule). Two elements may share 2, 4, or 6 bonding electrons (1, 2, or 3 line bonds). Include lone pair non bonding electrons. 1. S 2 - 3. Se 4. Cs 5. O 6. O 2 7. K 8. K+ 9. Ge 10. Kr 11. CH 4 12. NH 4+ 13. 7 N 14. N 2 15. NO 2 - 16. NCO- 17. CO 2 18. N 2 H 2 19. CH 4 O 20. NF 3 21. CH 2 O 22. N 2 H 4 23. CH 2 O 2 24. C 2 H 2 25. HCN 26. CO 32 - 27. NO 3 C. Draw the line bond formula for the polyatomic ions and compounds. A line in a line-bond formula represents each pair of bonding electrons. Dots represent non-bonding (lone pair) electrons. See F 2 example. Lewis Dot Line Bond
Lewis dot structure (Covalent Bonds) OCTET RULE EXCEPTIONS: H 2 H is stable with 2 e-(duet rule). Be may be stable with 4 e-. B may be stable with 6 e- Write the Lewis structure and line bond (octet rule exception) formulas for: 1. H 2 2. Be. F 2 3. BF 3 Elements containing d orbitals may (or may not) use them for bonding and, therefore, may (or may not) be surrounded 8 (or more) electrons. Example: NF 3 , PF 3 , SF 6, and PF 5 (but not NF 5 ) are covalent compounds. Question: Why not NF 5 ? Draw the Lewis structure and line bond formula for: 4. Br. F 5 5. Xe. F 2 6. SF 4 7. SO 42 - 8. Br. F 3 9. PF 5 10. I 3 - 11. PF 6 - 12. Cl. O 2 -
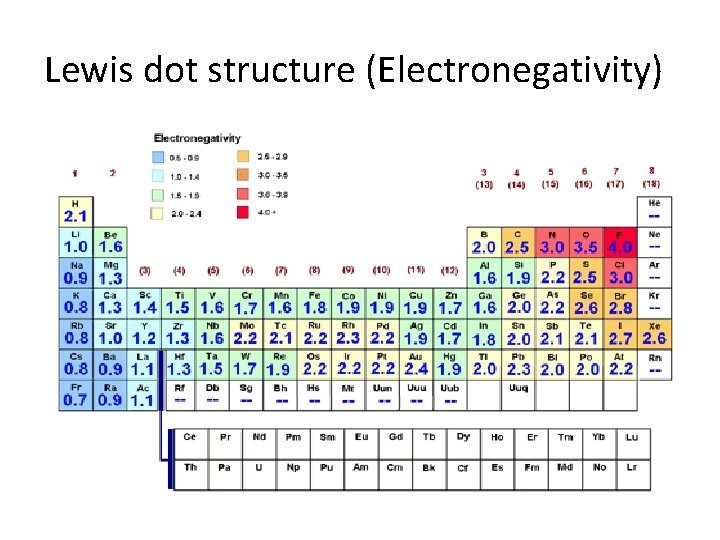
Lewis dot structure (Electronegativity)
Lewis dot structure (Covalent Bonds) A. Write at least 2 Lewis structures for the compounds below: Consider the duet rule, octet rule, and, multiple bonds, and octet exceptions. Write the Lewis dot structure and line bond formulas for the covalent compounds below. B. Calculate the formal charge for each non-hydrogen element in your Lewis dot structures. (FC = #valance e- - ½# bonding e- - # lone pair e- ) C. Lewis structures with formal charges of 0 are most likely to exist. Lewis structures with formal charges of + or - 1 ( -1 on the most electronegative element) might exist. Lewis structures with formal charges + or -2 (or more) do not exist and can be rejected. The sum of all formal charges equals the charge on the structure (0 for molecules, -1 for NO 2 - , or, +1 for NH 4+ ). See also Tro Page 381 - 382. 1. F 2 2. H 2 O 2 3. O 2 4. HCN 5. NO 2 - 6. NCO- * 7. NH 4+ 8. CO 2 9. CH 4 O 10. NO 3 - 11. HNO 3 12. C 2 H 2 F 2 13. CH 2 O 14. C 2 H 6 O ** 15. N 2 H 4 16. Cl. O 2 - 17. C 2 H 6 O 2 18. C 3 H 8 O 19. N 2 O 20. C 2 H 4 O 2 21. CO 32 - 22. SO 42*24. Look at the formal charges. Both NCO- (cyanate) and CNO‑ (fulminate) exist. Why would you predict fulminate to be less stable (more likely to explode) than cyanate? ** Both compounds are available as isomers.
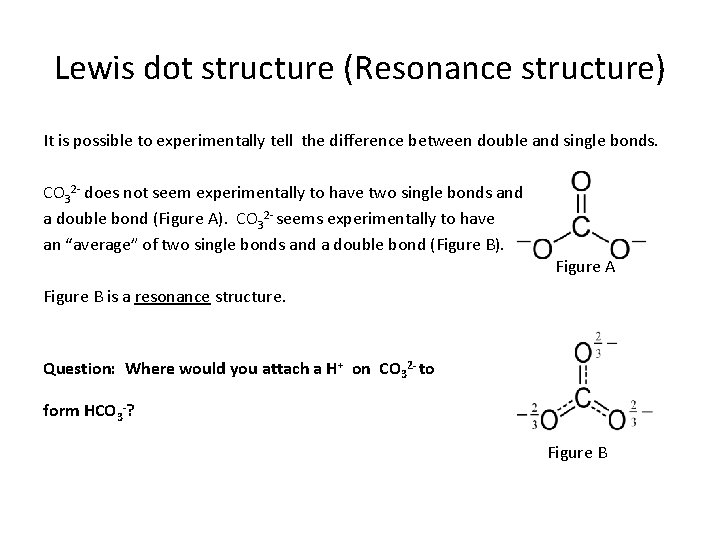
Lewis dot structure (Resonance structure) It is possible to experimentally tell the difference between double and single bonds. CO 32 - does not seem experimentally to have two single bonds and a double bond (Figure A). CO 32 - seems experimentally to have an “average” of two single bonds and a double bond (Figure B). Figure A Figure B is a resonance structure. Question: Where would you attach a H+ on CO 32 - to form HCO 3 -? Figure B
- Slides: 7