Ionic Bonds Attraction between ions of opposite charges

Ionic Bonds Attraction between ions of opposite charges to become stable • Forms solid salts • Rearranges e- (gain or lose) to become more stable

• Ions – charged atoms (gained or lost e-) – Formed from ve- only – Cations- formed when a metal atom loses e- forming a + ion + + – Anion – formed when a nonmetal atom gains e- to form a - ion -
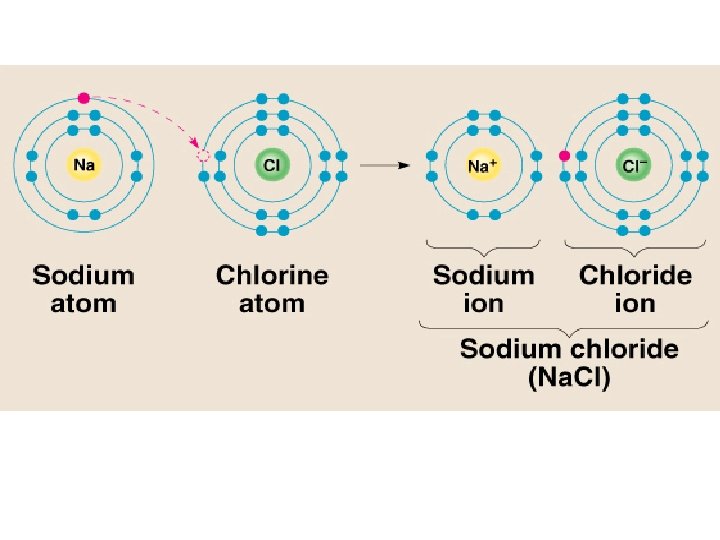
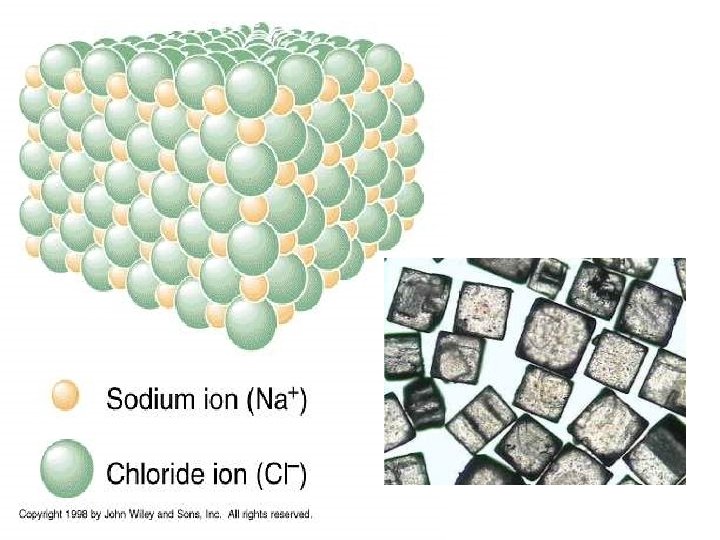

Ionic Bonds: One Big Greedy Thief Dog!

Writing Formulas and Naming Ionic Compounds RULES: • Write the symbol and charge of the cation • Write the symbol and charge of the anion • Check the charges they must = 0

Binary Ionic Compounds (2 atoms: metal & nonmetal) (1) sodium + iodine +1 -1 Na + I (2) calcium + oxygen +2 -2 Ca + O (3) potassium + sulfur +1 -2 K + S (4) aluminum + oxygen +3 -2 Al + O (5) Chlorine + magnesium +2 -1 Mg + Cl Na. I Ca. O K 2 S Al 2 O 3 Mg. Cl 2

Rules for Naming Binary Ionic compounds Write the name of the cation Write the root for the anion with the ending “ide” Ex: fluorine sulfur oxygen fluoride sulfide oxide
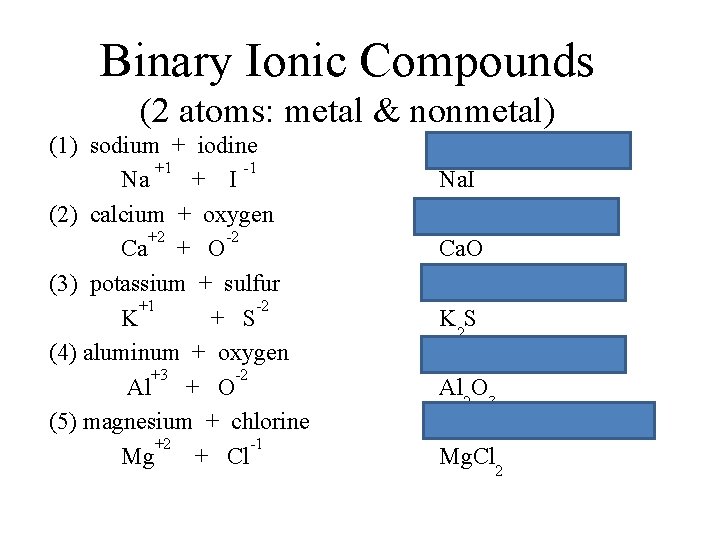
Binary Ionic Compounds (2 atoms: metal & nonmetal) (1) sodium + iodine +1 -1 Na + I (2) calcium + oxygen +2 -2 Ca + O (3) potassium + sulfur +1 -2 K + S (4) aluminum + oxygen +3 -2 Al + O (5) magnesium + chlorine +2 -1 Mg + Cl Sodium iodide Na. I calcium oxide Ca. O potassium sulfide K 2 S aluminum oxide Al 2 O 3 magnesium chloride Mg. Cl 2
- Slides: 9