Ionic Bonding Webquest and Ionic Compound Naming Objectives

Ionic Bonding Webquest and Ionic Compound Naming

• Objectives: – Differentiate between the properties of ionic and covalent bonds – Correctly apply naming rules to writing names for ionic compound formulas. • Assessment: – Formal Assessment • Analyzing student responses to the Naming Formulas Practice and the Bonding Webquest. Analyzing responses to the exit ticket. – Informal Assessment • Listening to student and group interactions while completing the naming practice and the Bonding Web. Quest • Common Core Connections – – – Demonstrate Independence Build Strong Content Knowledge Use technology and digital media strategically and capably Look for and make use of structure Look for and express regularity in repeated reasoning

Lesson Sequence • Warm – up • Evaluate: review Criss-Cross Method WS – Informal assessment • Explore/Explain/ Elaborate: Ionic Bonds Stations – Ionic Bonding Webquest – Naming Ionic Formulas Notes – Naming Ionic Formulas practice • Formal assessment • Exit Ticket – Formal Assessment
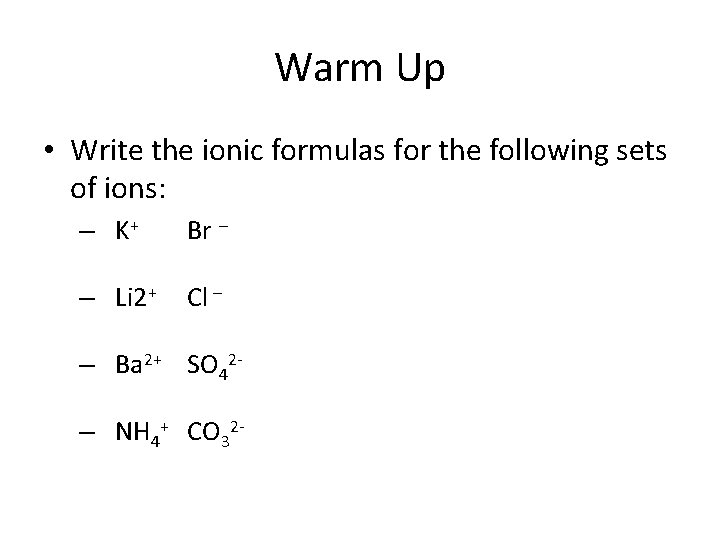
Warm Up • Write the ionic formulas for the following sets of ions: – K+ Br – – Li 2+ Cl – – Ba 2+ SO 42– NH 4+ CO 32 -

Objective • Today I will be able to: – Differentiate between the properties of ionic and covalent bonds – Correctly apply naming rules to writing names for ionic compound formulas.

Homework • Wear closed toe shoes tomorrow!! • Finish 7 -1, 7 -3 practice problems WS

Agenda • Warm – Up • Review Criss-Cross Method • Stations: – Ionic bonding webquest OR naming ionic formulas • Switch station to the one you have not been to – Ionic bonding webquest OR naming ionic formulas • Exit Ticket

Stations Directions • If you have an odd numbered popsicle stick, you will begin working on the Ionic Bonding Webquest. – Visit the link on the worksheet to answer the questions – No more than 3 people should be at one computer – The only website you are allowed to be on is the webquest • If you have an even number popsicle stick, you will begin with me. – We will begin by discussing how to name compounds – Then we will complete some practice worksheets • After 20 minutes we will switch stations

Naming Ionic Formulas

Naming Ionic Compounds • Standard Naming Rules – Write the full name of the metal first – Then write the second element, take off the ending and add the suffix –ide – If the second ion is a polyatomic ion, use the full name of the ion • Examples – Ca. O – Calcium Oxide – Ba. Cl 2 – Barium Chloride

Naming Ionic Compounds • Exceptions – Transition metals can have multiple oxidation numbers – Standard naming rules apply but we need to account for the oxidation number • use a roman numeral – Examples • Fe. Cl 3 – Iron(III) Chloride • Fe. Cl 2 – Iron (II) Chloride • Pb. Cl 4 – Lead(IV) Chloride

Naming Ionic Compounds Practice Complete the worksheet. Please ask Ms. Ose for help with questions.

What questions do you have about naming ionic formulas or the webquest? Whatever you did not finish in class will become your homework.

Exit Ticket • What is wrong with the names of these compounds? – Mg. SO 4 – Magnesium sulfide – Baride chlorine – Aluminum oxygen
- Slides: 14