Ionic Bonding Ionic Bonding Ionic compounds are a
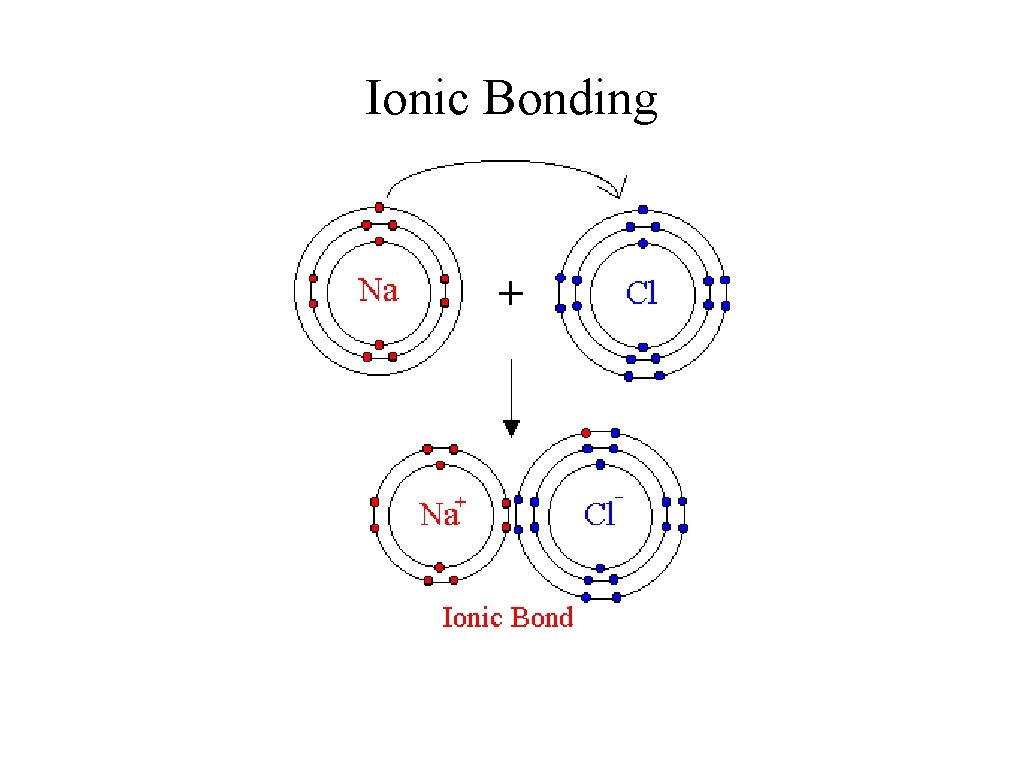
Ionic Bonding
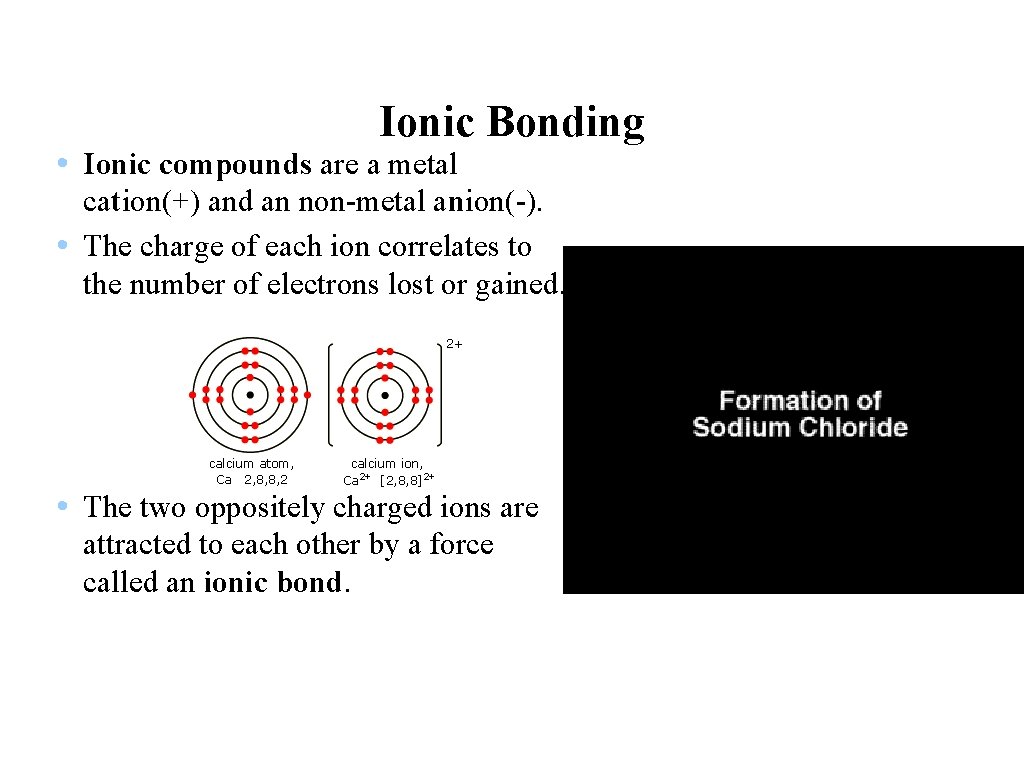
Ionic Bonding • Ionic compounds are a metal cation(+) and an non-metal anion(-). • The charge of each ion correlates to the number of electrons lost or gained. • The two oppositely charged ions are attracted to each other by a force called an ionic bond.

Ionic Properties / Structure • Ionic compounds are usually solids and form ionic crystals. These are more commonly known as salts

General properties • High melting point • Many are brittle • When ionic compounds are dissolved in water they conduct electricity.
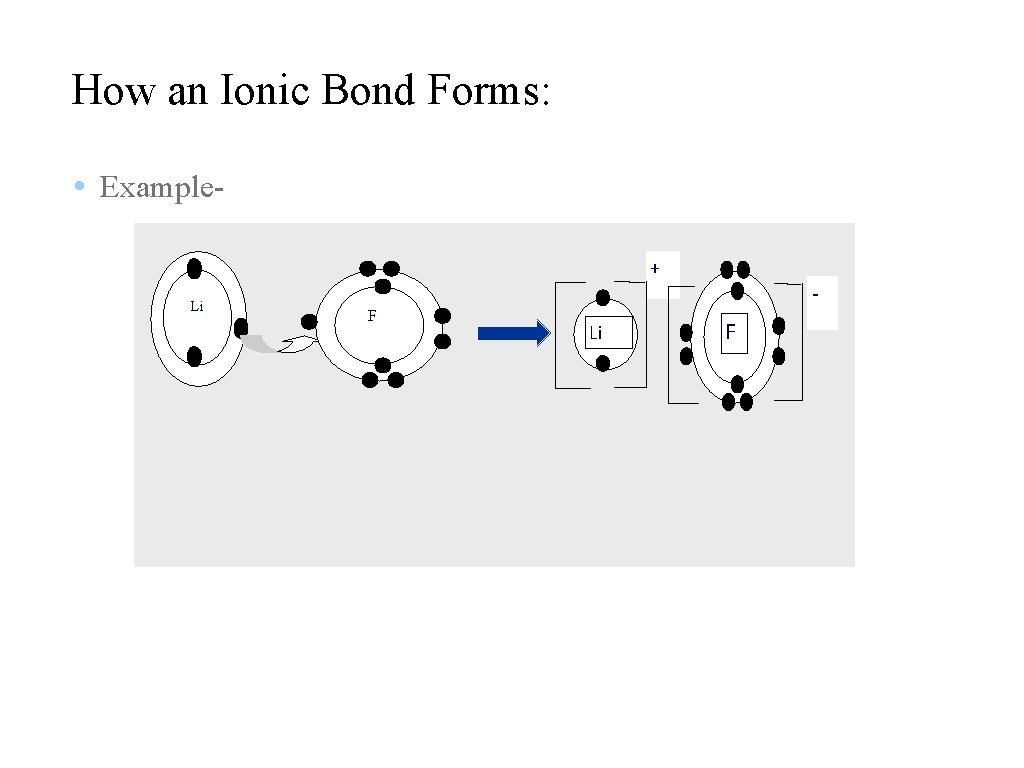
How an Ionic Bond Forms: • Example+ Li F
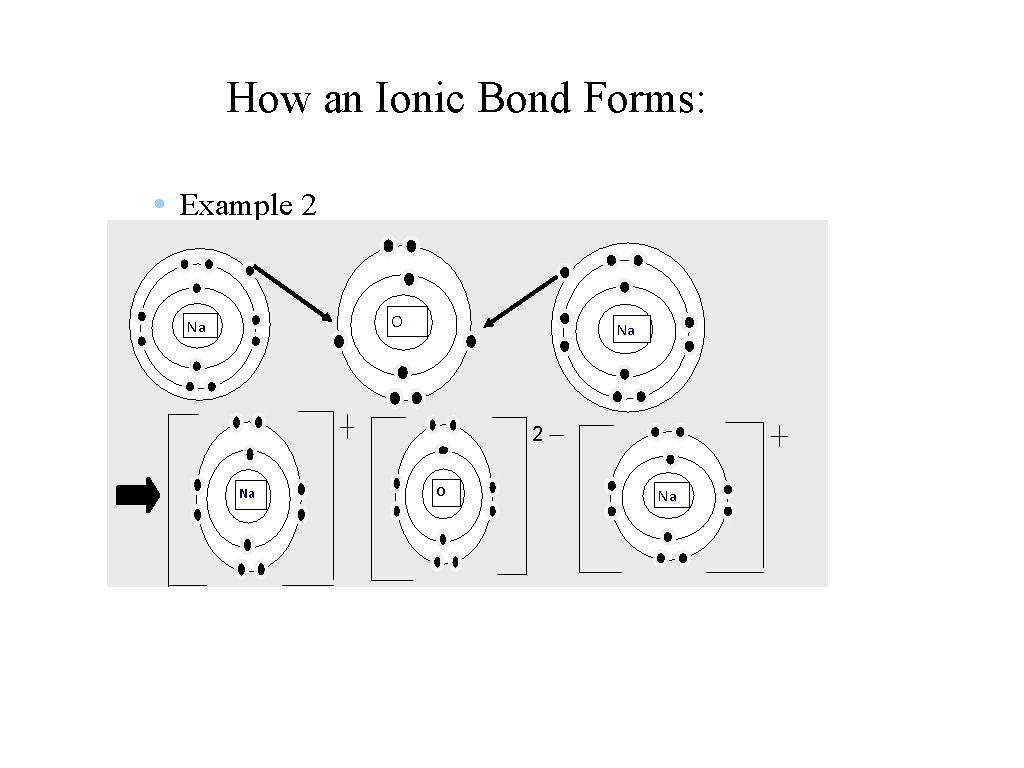
How an Ionic Bond Forms: • Example 2 O Na Na 2 Na O Na
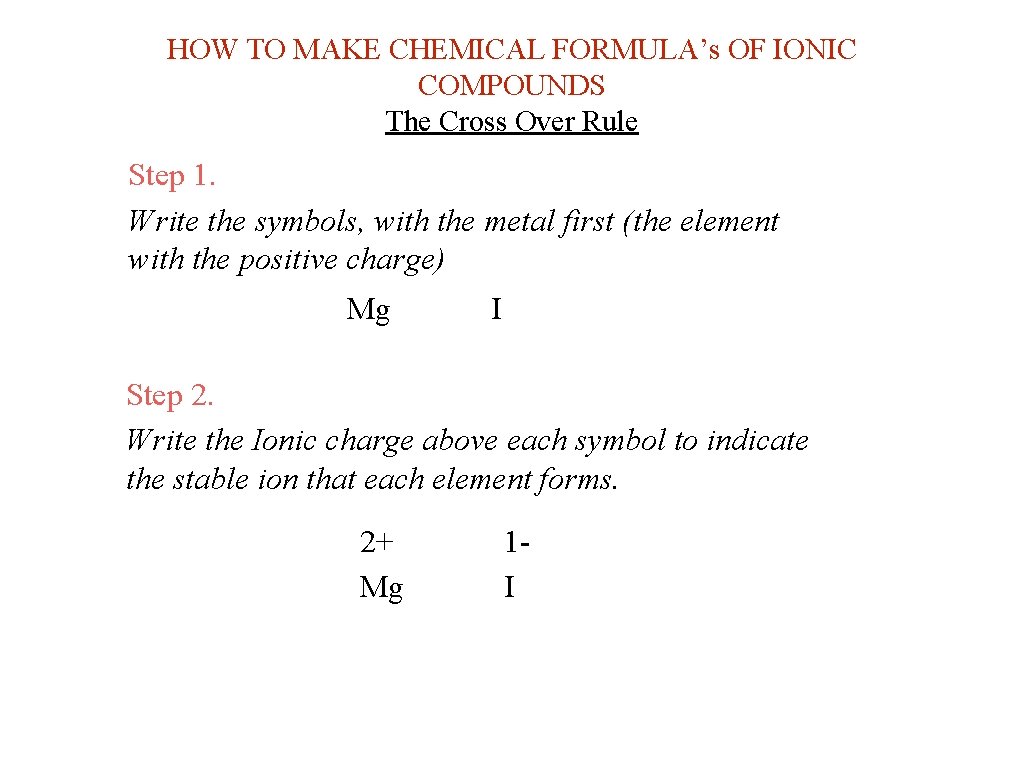
HOW TO MAKE CHEMICAL FORMULA’s OF IONIC COMPOUNDS The Cross Over Rule Step 1. Write the symbols, with the metal first (the element with the positive charge) Mg I Step 2. Write the Ionic charge above each symbol to indicate the stable ion that each element forms. 2+ Mg 1 I
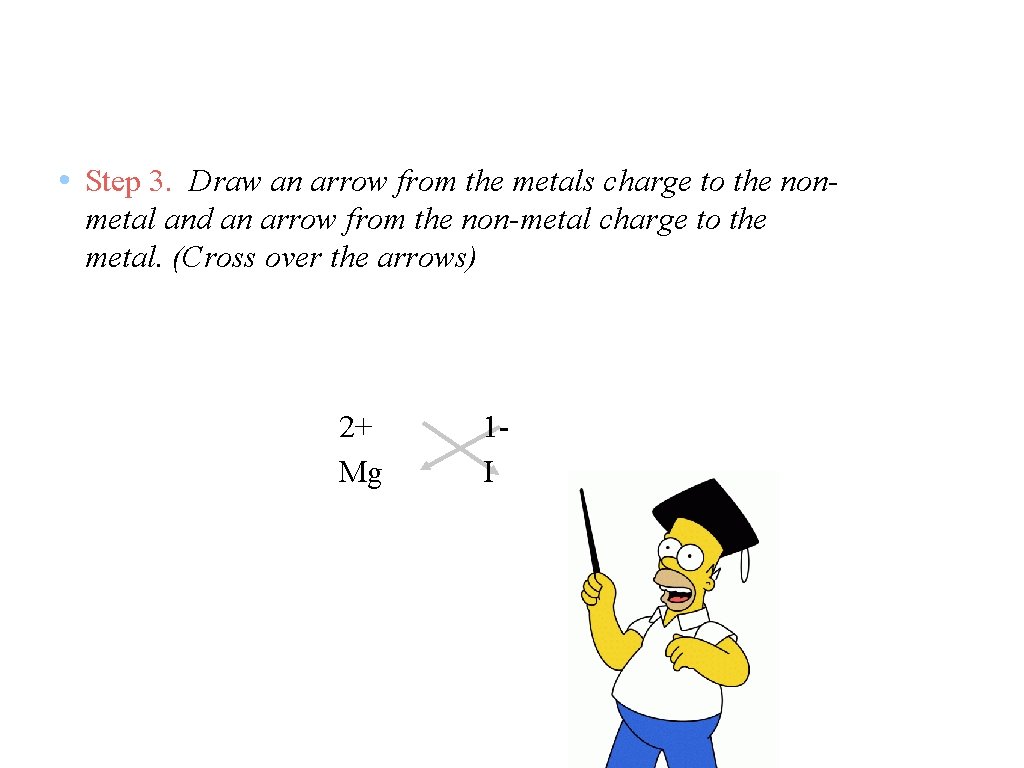
• Step 3. Draw an arrow from the metals charge to the nonmetal and an arrow from the non-metal charge to the metal. (Cross over the arrows) 2+ Mg 1 I

Step 4. Fill in the number of atoms from each element will have by following the arrows. If need be reduce to lowest terms. Mg. I 2 (if the number crossed is a 1, the 1 is not shown)

Example 2 - Try yourself Ca O

Example 3 - Try yourself Zr O

Example 4 - Try yourself Al S

Example 5 - Try yourself Sr N

Example 6 - Try yourself Mo P
IUPAC nomenclature ■ is a system of naming chemical compounds. ■ It is maintained by the International Union of Pure and Applied Chemistry.
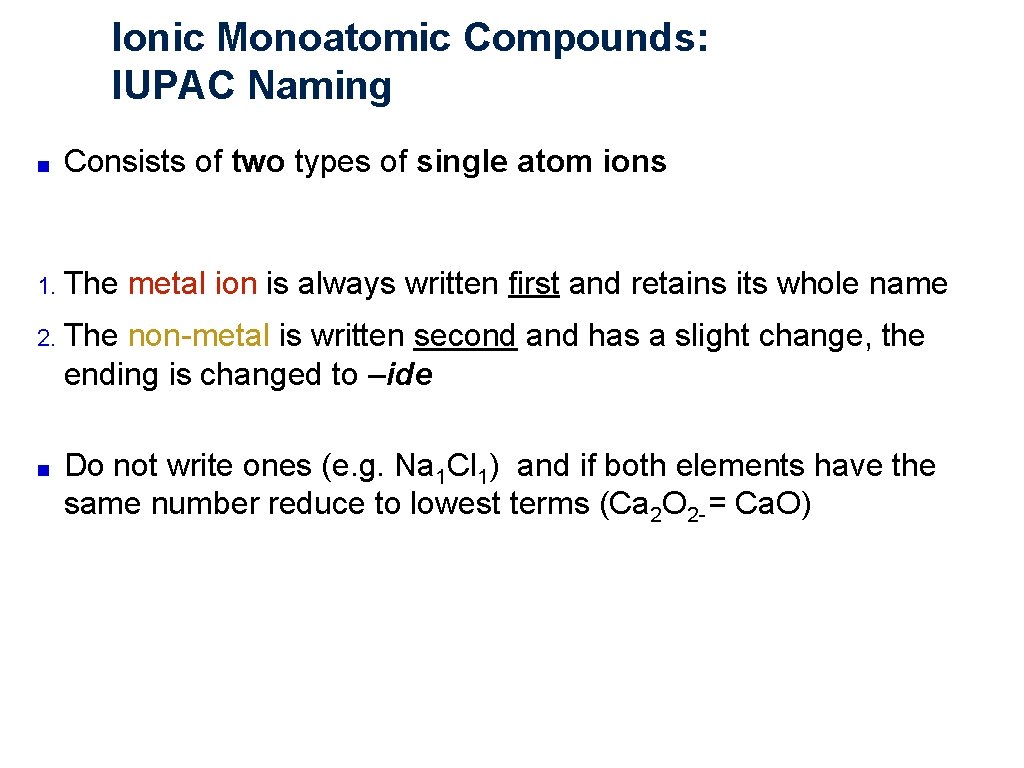
Ionic Monoatomic Compounds: IUPAC Naming ■ Consists of two types of single atom ions 1. The metal ion is always written first and retains its whole name 2. The non metal is written second and has a slight change, the ending is changed to –ide ■ Do not write ones (e. g. Na 1 Cl 1) and if both elements have the same number reduce to lowest terms (Ca 2 O 2 = Ca. O)
- Slides: 16