Ionic Bonding Illustrating the bonding of Sodium and
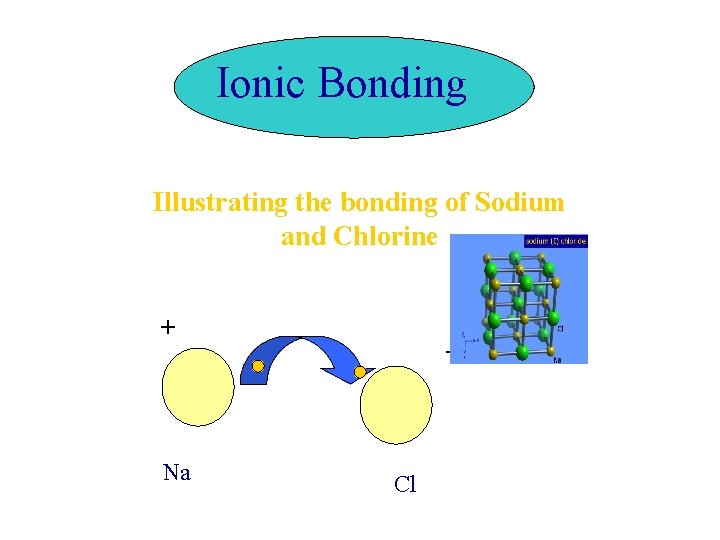
Ionic Bonding Illustrating the bonding of Sodium and Chlorine + Na - Cl
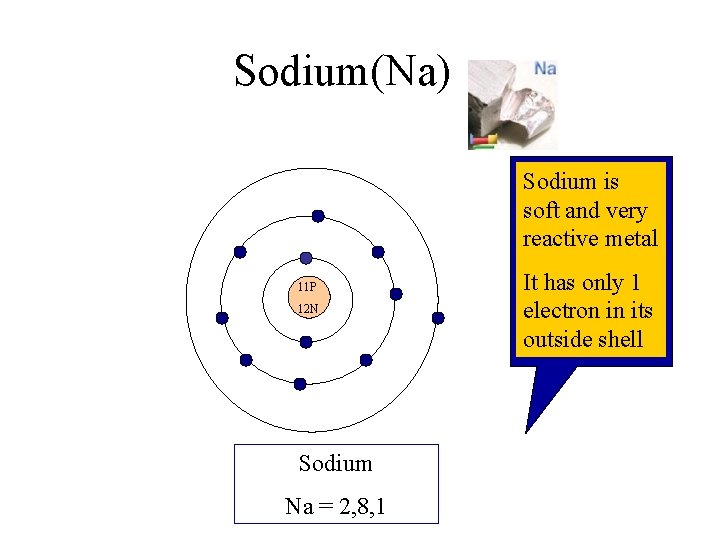
Sodium(Na) Sodium is soft and very reactive metal 11 P 12 N Sodium Na = 2, 8, 1 It has only 1 electron in its outside shell
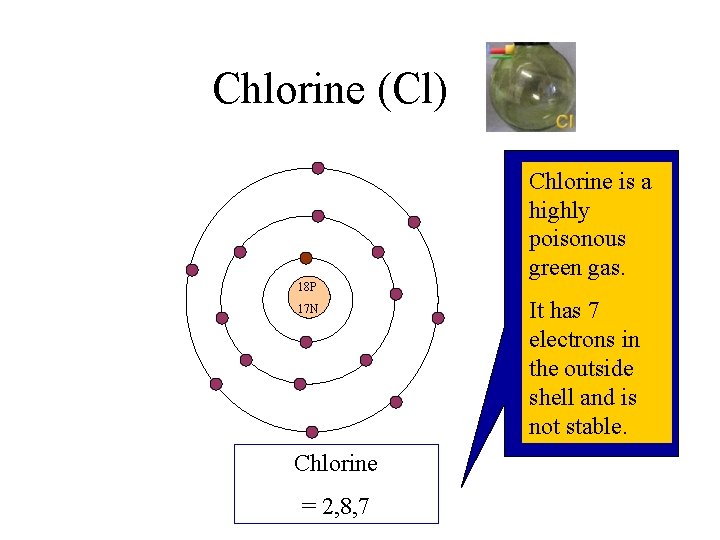
Chlorine (Cl) Chlorine is a highly poisonous green gas. 18 P 17 N Chlorine = 2, 8, 7 It has 7 electrons in the outside shell and is not stable.
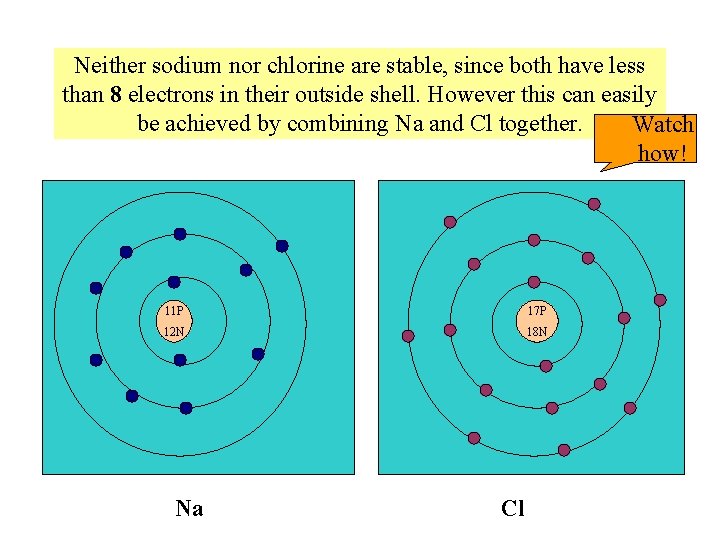
Neither sodium nor chlorine are stable, since both have less than 8 electrons in their outside shell. However this can easily be achieved by combining Na and Cl together. Watch how! 11 P 17 P 12 N 18 N Na Cl
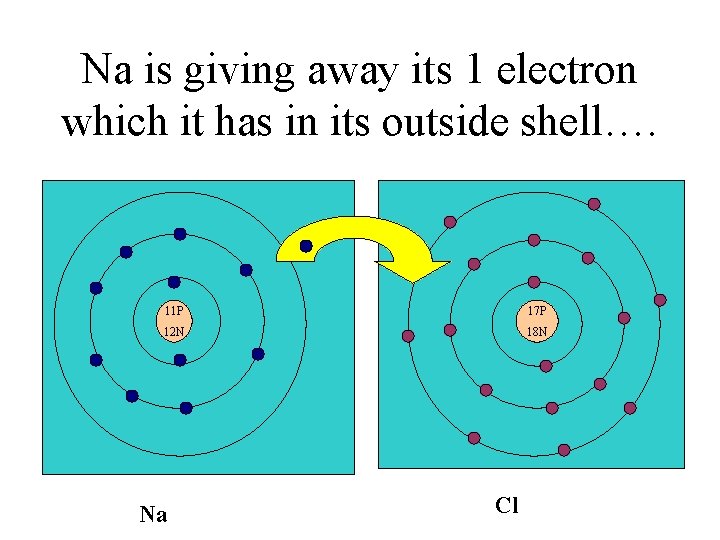
Na is giving away its 1 electron which it has in its outside shell…. 11 P 17 P 12 N 18 N Na Cl
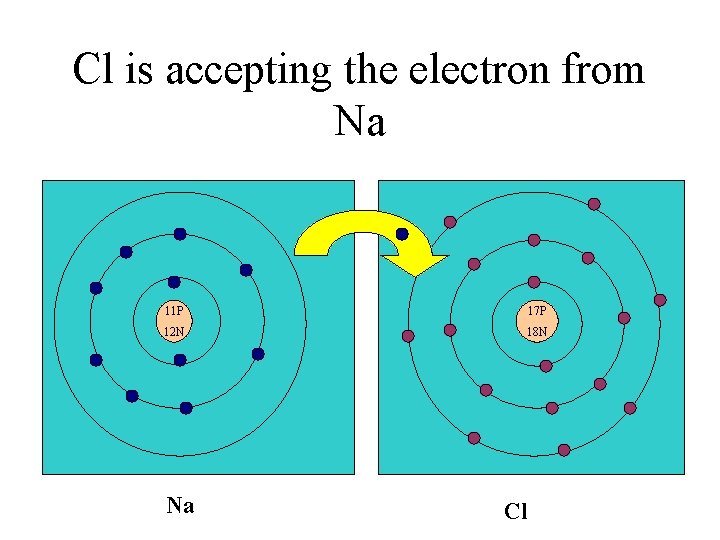
Cl is accepting the electron from Na 11 P 17 P 12 N 18 N Na Cl
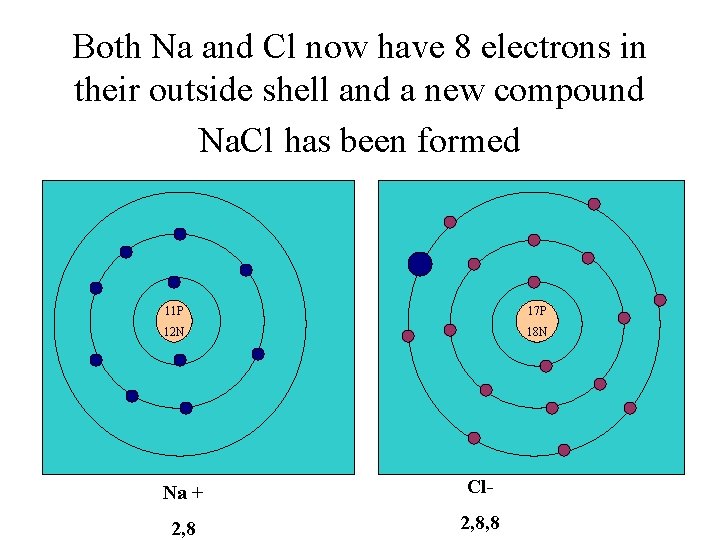
Both Na and Cl now have 8 electrons in their outside shell and a new compound Na. Cl has been formed 11 P 17 P 12 N 18 N Na + Cl- 2, 8, 8
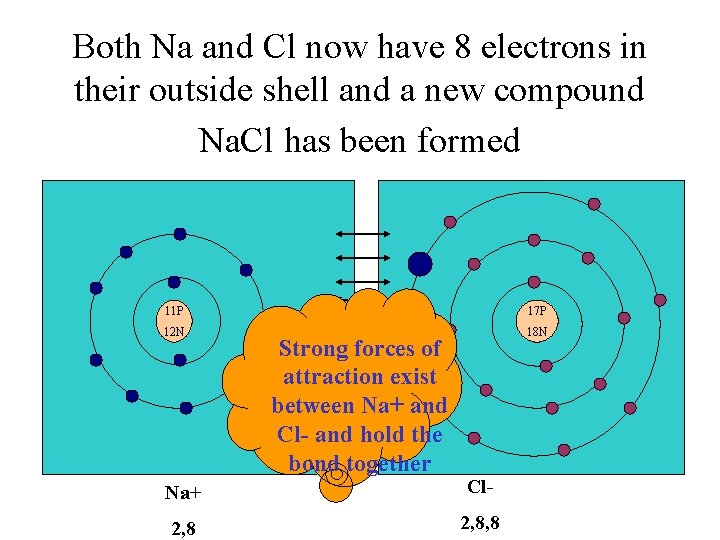
Both Na and Cl now have 8 electrons in their outside shell and a new compound Na. Cl has been formed 11 P 17 P 12 N 18 N Strong forces of attraction exist between Na+ and Cl- and hold the bond together Na+ Cl- 2, 8, 8

Satisfying the Octet Rule…. . The Octet Rule: The Octet rule is simply a rule which helps us to understand bonding Atoms bond together so that each atom attains an electron arrangement of 8 electrons in its outermost shell.
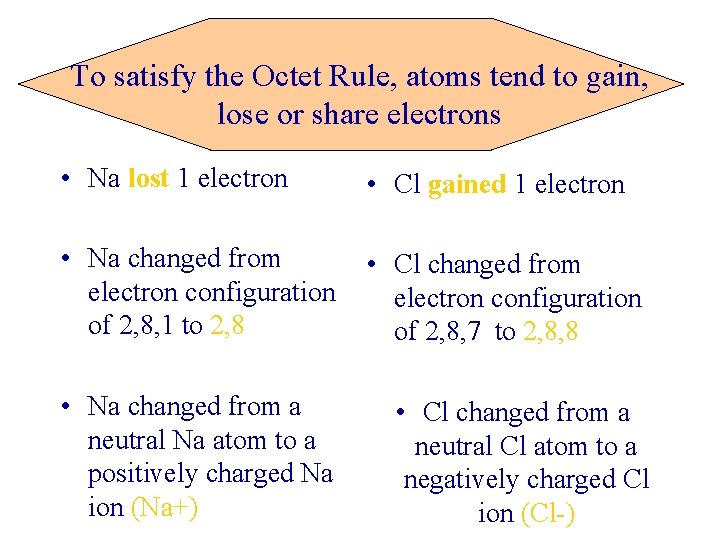
To satisfy the Octet Rule, atoms tend to gain, lose or share electrons • Na lost 1 electron • Cl gained 1 electron • Na changed from electron configuration of 2, 8, 1 to 2, 8 • Cl changed from electron configuration of 2, 8, 7 to 2, 8, 8 • Na changed from a neutral Na atom to a positively charged Na ion (Na+) • Cl changed from a neutral Cl atom to a negatively charged Cl ion (Cl-)
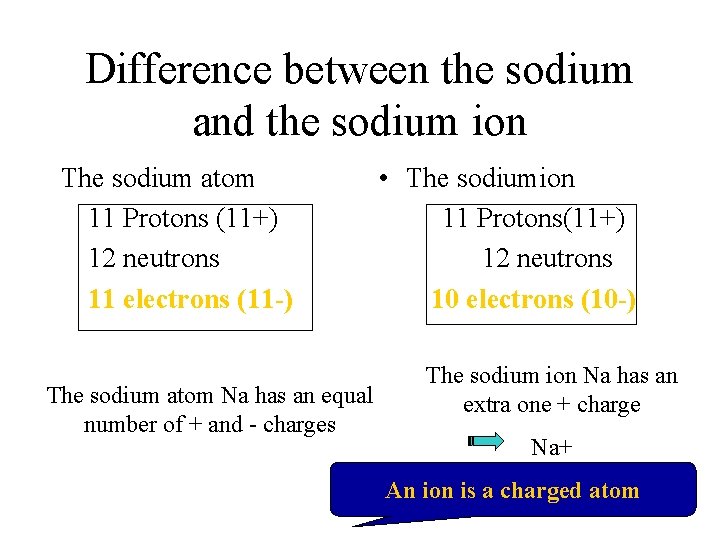
Difference between the sodium and the sodium ion The sodium atom 11 Protons (11+) 12 neutrons 11 electrons (11 -) The sodium atom Na has an equal number of + and - charges • The sodiumion 11 Protons(11+) 12 neutrons 10 electrons (10 -) The sodium ion Na has an extra one + charge Na+ An ion is a charged atom
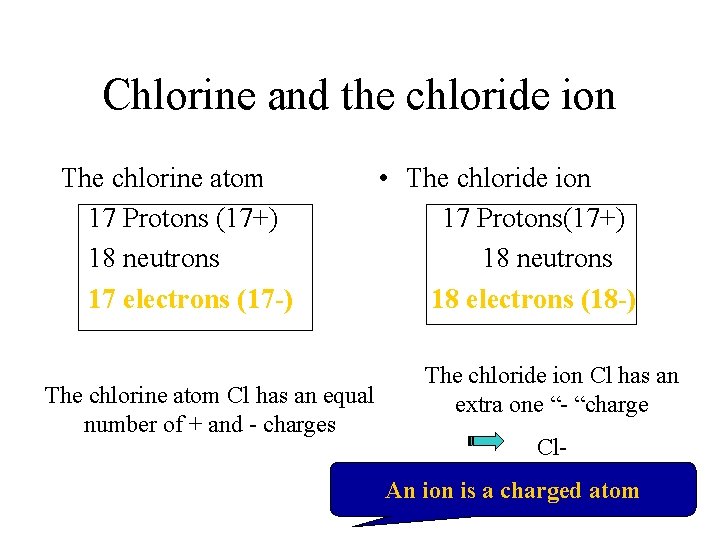
Chlorine and the chloride ion The chlorine atom 17 Protons (17+) 18 neutrons 17 electrons (17 -) The chlorine atom Cl has an equal number of + and - charges • The chloride ion 17 Protons(17+) 18 neutrons 18 electrons (18 -) The chloride ion Cl has an extra one “- “charge Cl. An ion is a charged atom
- Slides: 12