Ionic Bonding Ch 8 CVHS Chemistry EQ How
Ionic Bonding Ch 8 CVHS Chemistry EQ: How does the electron configuration of elements impact the Ionic Bonding?
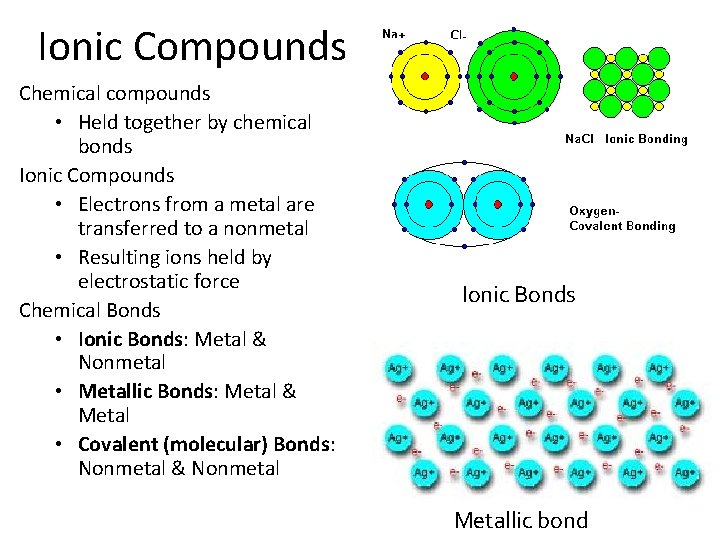
Ionic Compounds Chemical compounds • Held together by chemical bonds Ionic Compounds • Electrons from a metal are transferred to a nonmetal • Resulting ions held by electrostatic force Chemical Bonds • Ionic Bonds: Metal & Nonmetal • Metallic Bonds: Metal & Metal • Covalent (molecular) Bonds: Nonmetal & Nonmetal Ionic Bonds Metallic bond
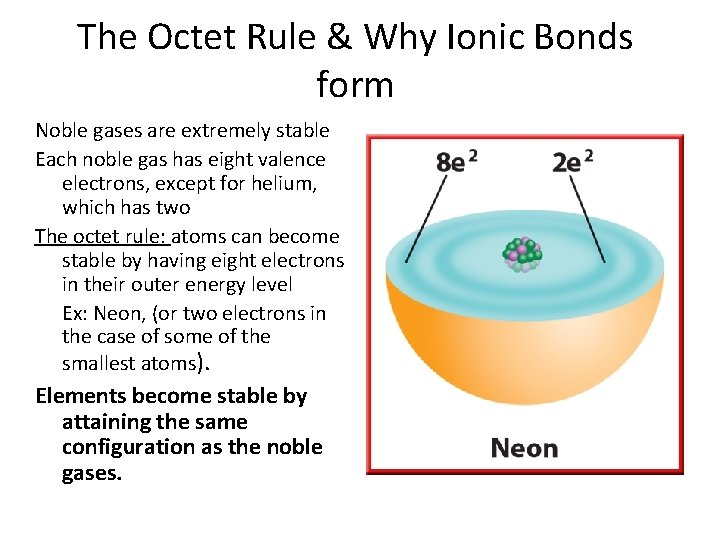
The Octet Rule & Why Ionic Bonds form Noble gases are extremely stable Each noble gas has eight valence electrons, except for helium, which has two The octet rule: atoms can become stable by having eight electrons in their outer energy level Ex: Neon, (or two electrons in the case of some of the smallest atoms). Elements become stable by attaining the same configuration as the noble gases.

The Octet Rule
Properties of ionic compounds • Practice Problem: Well organized, tightly bound ions w/ a crystal lattice structure. Crystalline solids @ room temp. so it takes a lot of energy to melt them to break the bonds Electrolytes: dissolve in water or melt and then conduct electricity – What bond would form between Ca and Br? – Answer: Ca. Br 2 – Ca (4 s 2) + Br (4 s 2 4 p 5) – 2 Br atoms take the 2 electrons from Ca and then both atoms have a noble gas configuration
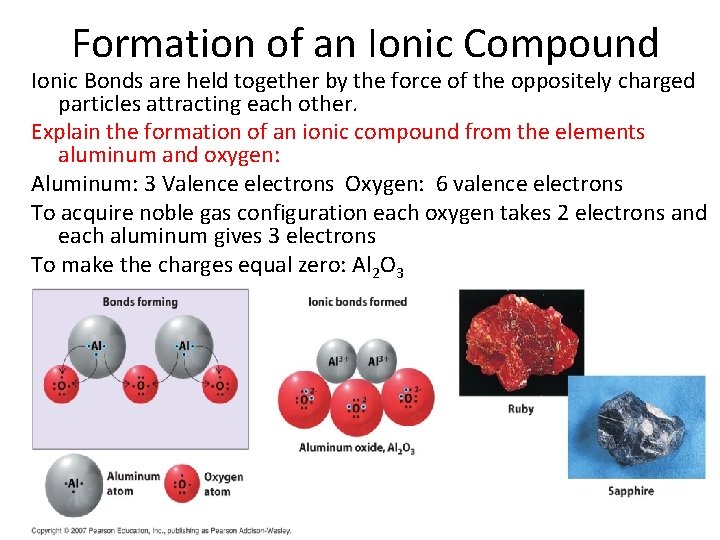
Formation of an Ionic Compound Ionic Bonds are held together by the force of the oppositely charged particles attracting each other. Explain the formation of an ionic compound from the elements aluminum and oxygen: Aluminum: 3 Valence electrons Oxygen: 6 valence electrons To acquire noble gas configuration each oxygen takes 2 electrons and each aluminum gives 3 electrons To make the charges equal zero: Al 2 O 3
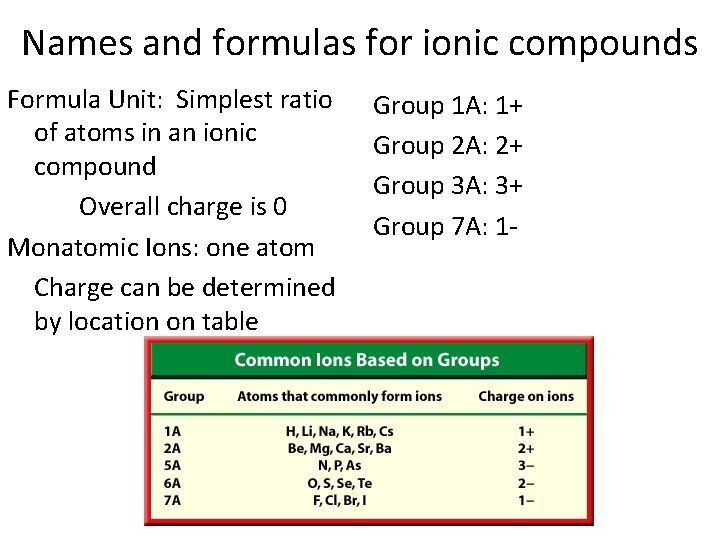
Names and formulas for ionic compounds Formula Unit: Simplest ratio of atoms in an ionic compound Overall charge is 0 Monatomic Ions: one atom Charge can be determined by location on table Group 1 A: 1+ Group 2 A: 2+ Group 3 A: 3+ Group 7 A: 1 -
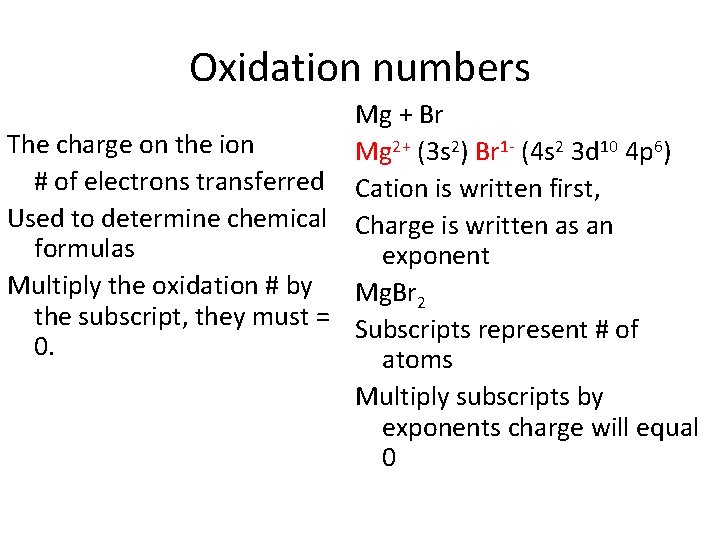
Oxidation numbers Mg + Br The charge on the ion Mg 2+ (3 s 2) Br 1 - (4 s 2 3 d 10 4 p 6) # of electrons transferred Cation is written first, Used to determine chemical Charge is written as an formulas exponent Multiply the oxidation # by Mg. Br 2 the subscript, they must = Subscripts represent # of 0. atoms Multiply subscripts by exponents charge will equal 0
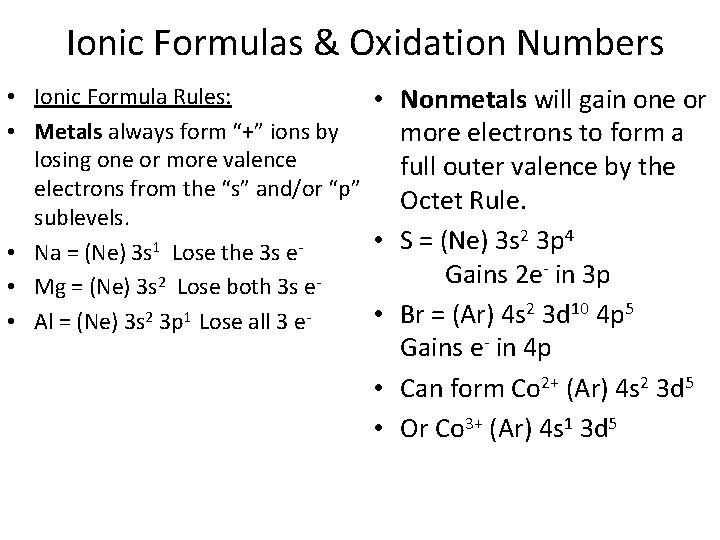
Ionic Formulas & Oxidation Numbers • Ionic Formula Rules: • Nonmetals will gain one or • Metals always form “+” ions by more electrons to form a losing one or more valence full outer valence by the electrons from the “s” and/or “p” Octet Rule. sublevels. 2 3 p 4 • S = (Ne) 3 s 1 • Na = (Ne) 3 s Lose the 3 s e - in 3 p Gains 2 e 2 • Mg = (Ne) 3 s Lose both 3 s e 2 3 d 10 4 p 5 2 1 • Br = (Ar) 4 s • Al = (Ne) 3 s 3 p Lose all 3 e Gains e- in 4 p • Can form Co 2+ (Ar) 4 s 2 3 d 5 • Or Co 3+ (Ar) 4 s 1 3 d 5
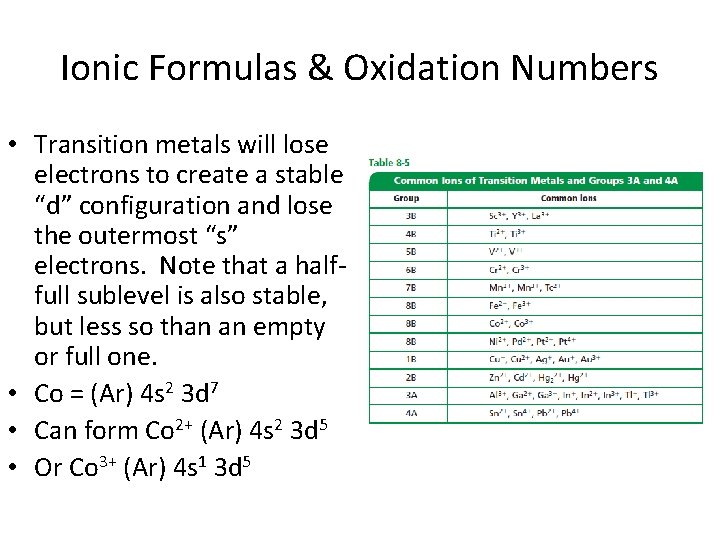
Ionic Formulas & Oxidation Numbers • Transition metals will lose electrons to create a stable “d” configuration and lose the outermost “s” electrons. Note that a halffull sublevel is also stable, but less so than an empty or full one. • Co = (Ar) 4 s 2 3 d 7 • Can form Co 2+ (Ar) 4 s 2 3 d 5 • Or Co 3+ (Ar) 4 s 1 3 d 5
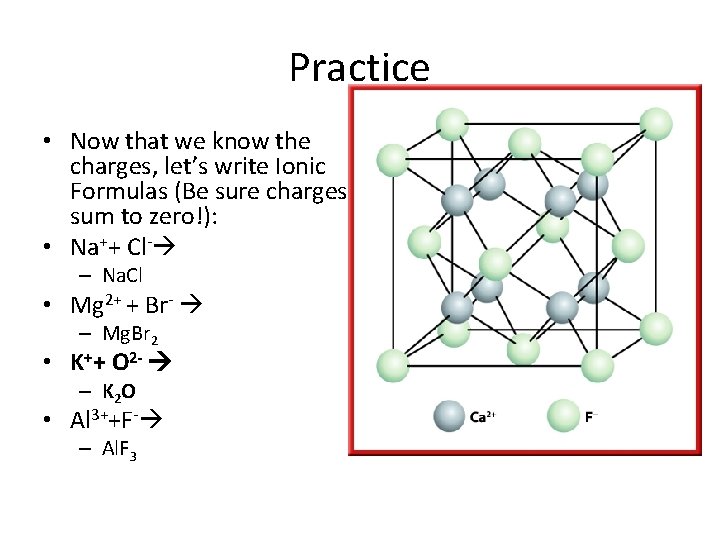
Practice • Now that we know the charges, let’s write Ionic Formulas (Be sure charges sum to zero!): • Na++ Cl- – Na. Cl • Mg 2+ + Br- – Mg. Br 2 • K++ O 2 - – K 2 O • Al 3++F- – Al. F 3
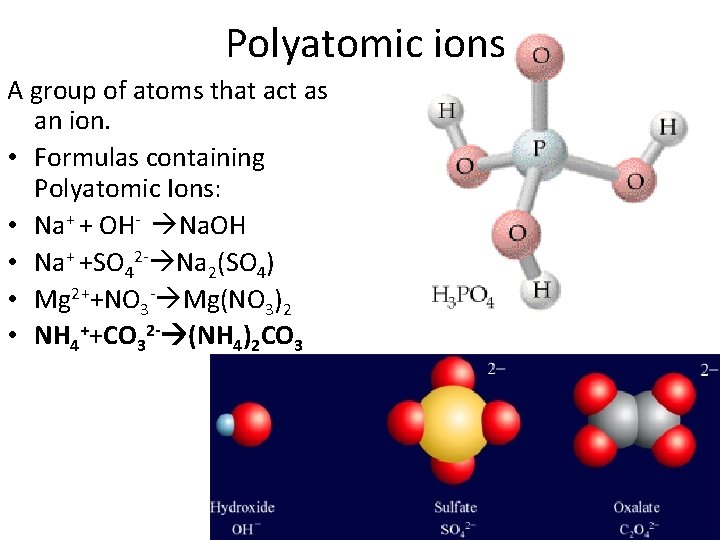
Polyatomic ions A group of atoms that act as an ion. • Formulas containing Polyatomic Ions: • Na+ + OH- Na. OH • Na+ +SO 42 - Na 2(SO 4) • Mg 2++NO 3 - Mg(NO 3)2 • NH 4++CO 32 - (NH 4)2 CO 3
- Slides: 12