Ionic Bonding and Writing Formulas Footer Text 192022

Ionic Bonding and Writing Formulas Footer Text 1/9/2022 1
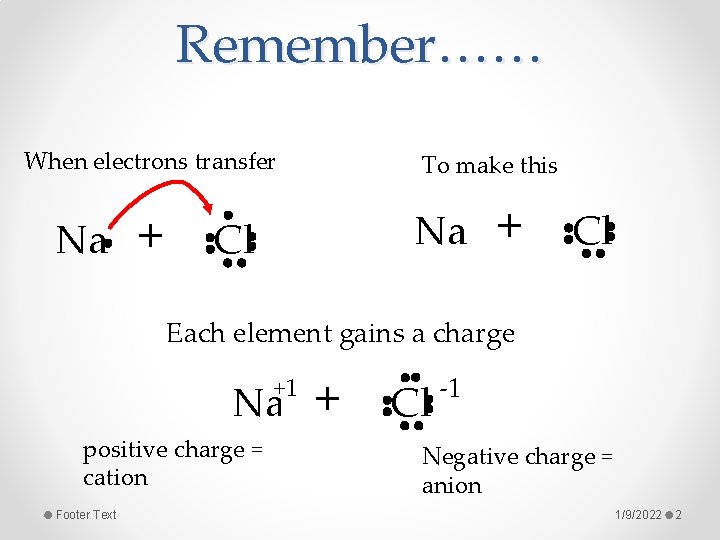
Remember…… When electrons transfer Na + To make this Na Cl + Cl Each element gains a charge +1 Na positive charge = cation Footer Text + Cl -1 Negative charge = anion 1/9/2022 2
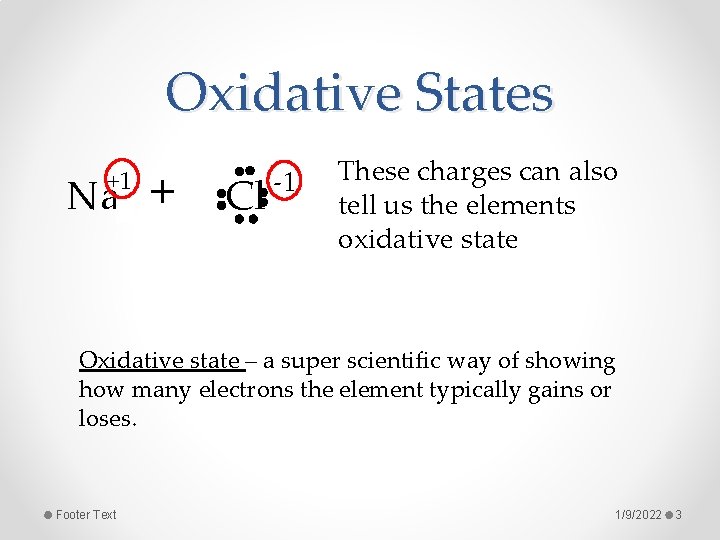
Oxidative States +1 Na + Cl -1 These charges can also tell us the elements oxidative state Oxidative state – a super scientific way of showing how many electrons the element typically gains or loses. Footer Text 1/9/2022 3

Oxidative State We can tell the oxidative state of many elements by looking at the periodic table!!!! Footer Text 1/9/2022 4
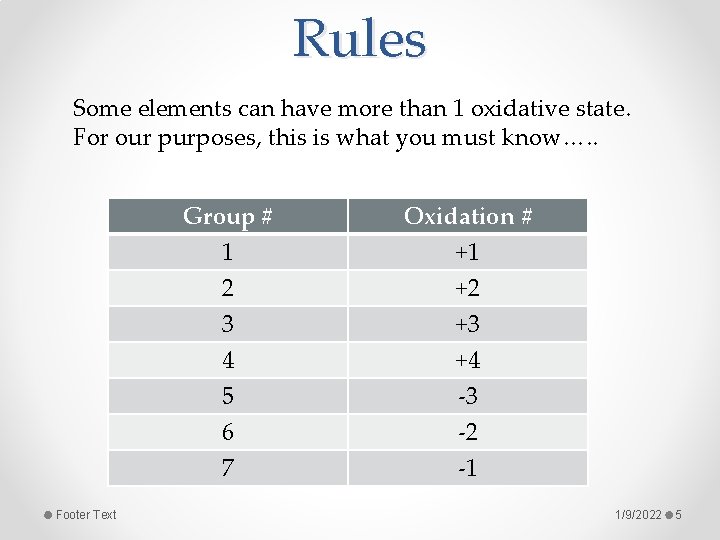
Rules Some elements can have more than 1 oxidative state. For our purposes, this is what you must know…. . Footer Text Group # 1 2 3 Oxidation # +1 +2 +3 4 5 6 7 +4 -3 -2 -1 1/9/2022 5

Shortcut!! Oxidation numbers are good to know. They are a means to a shortcut for writing formulas, instead of drawing Lewis Dot Structures. For Example: Aluminum + Chloride Footer Text 1/9/2022 6

Shortcut !!!! Aluminum + Chloride First, determine the oxidation number for each element and write it at the top right corner of the element Footer Text 1/9/2022 7

Shortcut!!!! Aluminum + Chloride Footer Text Group # 1 2 3 Oxidation # +1 +2 +3 4 5 6 7 +4 -3 -2 -1 1/9/2022 8

Shortcut!!! Al+3 + Cl-1 Now, criss cross applesauce!! Al+3 + Cl-1 Cross the numbers only, and write them at the bottom, as subscripts!! Footer Text 1/9/2022 9

Shortcut!!! Al. Cl 3 Now, just for kicks, lets do it using Lewis Dot structures to see if we get the same thing Footer Text 1/9/2022 10
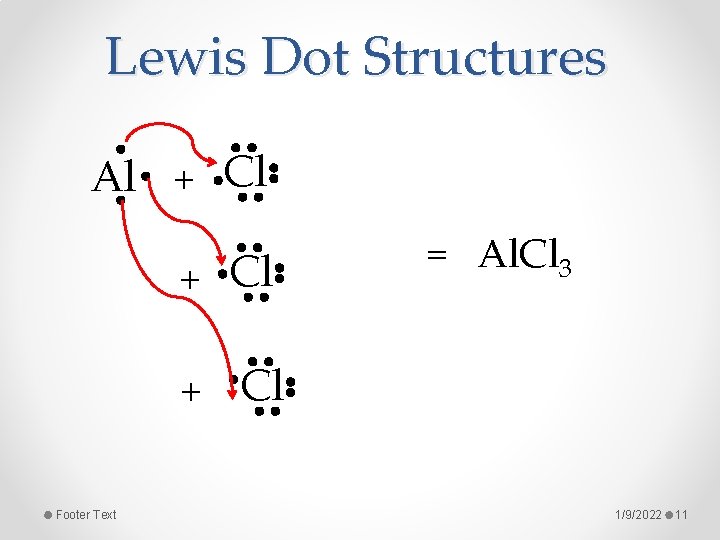
Lewis Dot Structures Al + Cl + Footer Text = Al. Cl 3 Cl 1/9/2022 11
- Slides: 11