IONIC BONDING AND IONIC FORMULAS Vocabulary bond ionic

IONIC BONDING AND IONIC FORMULAS Vocabulary • bond • ionic bond • electrostatic attraction • subscript • formula “Spiral Jetty”, Robert Smithson, American, 1970

PREDICTION • What types of particles do you think are involved in ionic bonding? • What are the two types of ions? • How is each type of ion formed?

CHEMICAL BOND • What is a bond? • An attractive force holding two or more atoms together. • How do you think ions could form a bond?
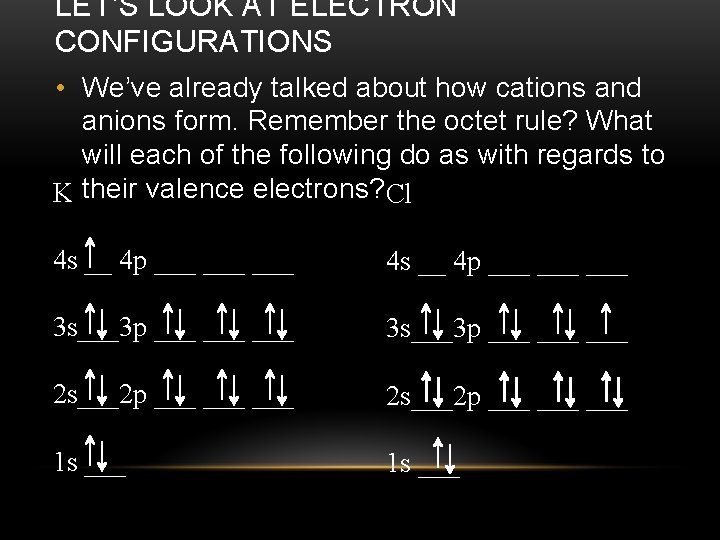
LET’S LOOK AT ELECTRON CONFIGURATIONS • We’ve already talked about how cations and anions form. Remember the octet rule? What will each of the following do as with regards to K their valence electrons? Cl 4 s __ 4 p ___ ___ ___ 3 s___3 p ___ ___ ___ 2 s___2 p ___ ___ ___ 1 s ___

LET’S LOOK AT ELECTRON CONFIGURATIONS • In ionic bonding, two elements are involved: one that will lose electrons to form a cation and one that will gain electrons to form an anion. One element will lose one or more electrons to the other element. K (will lose e-) Cl (will gain e-) 4 s __ 4 p ___ ___ ___ 3 s___3 p ___ ___ ___ 2 s___2 p ___ ___ ___ 1 s ___

LET’S LOOK AT ELECTRON CONFIGURATIONS • In ionic bonding, two elements are involved: one that will lose electrons to form a cation and one that will gain electrons to form an anion. One element will lose one or more electrons to the other element. K+ Cl- 4 s __ 4 p ___ ___ ___ 3 s___3 p ___ ___ ___ 2 s___2 p ___ ___ ___ 1 s ___

LET’S LOOK AT ELECTRON CONFIGURATIONS • Now that there is a positive ion and a negative ion, they can form a bond through electrostatic attraction. K+ Cl - This attraction IS the ionic bond! The chemical formula for this compound is KCl.

IMPORTANT POINTS ABOUT IONIC BONDING • One element must lose electrons (a metal) and one element must gain electrons (a non-metal). • The attraction of the resulting ions is the ionic bond. • The compound MUST be neutral (the sum of the positive and negative charges MUST add up to zero. )

EXAMPLE 1 • Let’s look at another example. What will the formula be for a compound with calcium and fluorine? First, draw the Lewis Structures for calcium and for fluorine. C a F
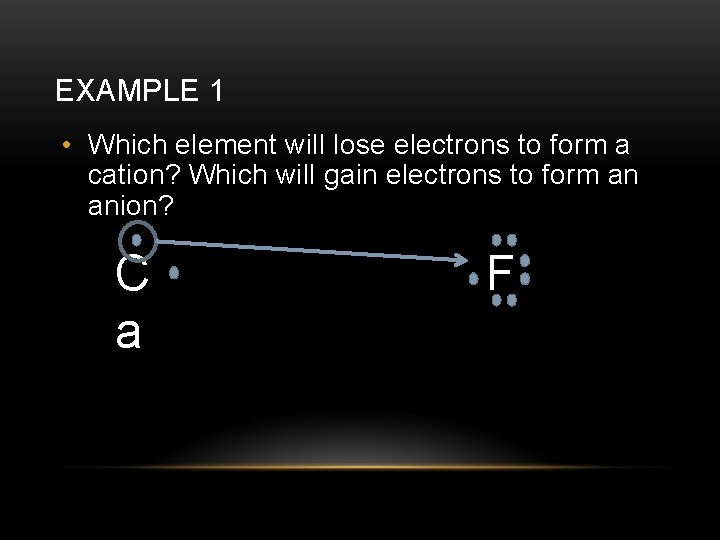
EXAMPLE 1 • Which element will lose electrons to form a cation? Which will gain electrons to form an anion? C a F
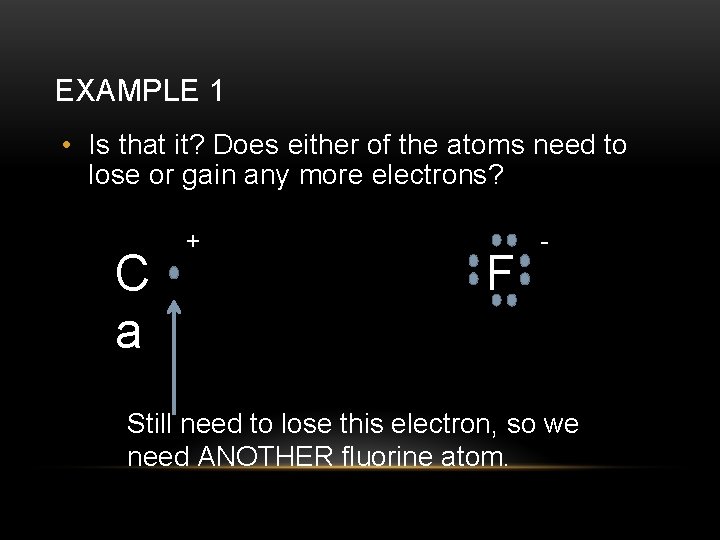
EXAMPLE 1 • Is that it? Does either of the atoms need to lose or gain any more electrons? C a + F - Still need to lose this electron, so we need ANOTHER fluorine atom.

EXAMPLE 1 • The number of atoms available to achieve a neutral compound is unlimited! C a + F F -

EXAMPLE 1 • So now all atoms are following the octet rule and the overall charge is zero. The atoms will now attract each other and form ionic bonds. C a 2+ F F - -

EXAMPLE 1 • So notice that the overall compound is neutral. Compounds must always be neutral! The chemical formula for this compound is Ca. F 2. - 2+ - C F a Lewis structure for the When drawing the F overall ionic compound, place brackets around the negative ions to indicate that the electrons belong completely to the negative ion and are not being shared

EXAMPLE 2 • Let’s try one now without drawing Lewis structures. • What will be the chemical formula for a compound containing lithium and phosphide (-ide means negative ion)?

EXAMPLE 2 • First, using the periodic table find out the charge of ions of each element. + Li 3 - P

EXAMPLE 2 • How many of each ion will be required to make a neutral compound? (i. e. , how many lithium ions will you need to neutralize the charge of the phosphorous ion? ) + Li 3 - P So the formula for this compound will be Li 3 P.

EXAMPLE 2 In the formula, Li 3 P, the 3 is a subscript. It tells you the number of lithium ions in the formula. There is also a subscript on P, but it is an understood 1. 1 is the only subscript that is not written.

EXAMPLE 3 • What is the formula for compounds containing each of the following pairs of ions? • rubidium and sulfide • strontium and oxide • aluminum and oxalate (-ate and –ite indicate a negative polyatomic ion)

EXAMPLE 3 • What is the formula for compounds containing each of the following pairs of ions? • Rb 2 S • Sr. O • Al 2(C 2 O 4)3
EXAMPLE 4 • Elements in the transition metals (the dblock) are multivalent meaning that they can have different charges. If an ionic compound contains a transition metal, you must be told what the charge of the element is in the particular compound.

EXAMPLE 4 • For instance, iron can have a 2+ charge or a 3+ charge. For a particular compound, you would have to be told which charge an iron ion had. • You can represent the ion with its charge in two different ways: 1. The symbol with the charge: Fe 2+ or Fe 3+ 2. The name of the ion with a Roman numeral in parentheses to indicate the charge:

EXAMPLE 4 • Write the formulas for each of the following compounds containing the ions indicated: • Fe 2+ and I • copper(I) and phosphide • phosphate and manganese(VI)

EXAMPLE 4 • Write the formulas for each of the following compounds containing the ions indicated: • Fe. I 2 • Cu 3 P • Mn(PO 4)2 (use parentheses when there are more then one polyatomic ion in the formula)

EXAMPLE 5 • There are some exceptions in the transition metals that only contain 1 charge so you do not use a Roman numeral to indicate their charge (yes, you need to know them): • silver is always 1+ • zinc is always 2+ • There are some elements that are not in the transition metals that have multiple charges so you must use a Roman numeral to indicate their charge (yes, you need to know them): • lead and tin can both be either 2+ or 4+

EXAMPLE 5 • Write the formulas for each of the following compounds containing the ions indicated: • silver and nitride • zinc and sulfide • lead(IV) and phosphide • iodide and tin(II)

EXAMPLE 5 • Write the formulas for each of the following compounds containing the ions indicated: • Ag 3 N • Zn. S • Pb 3 P 4 • Sn. I 2

THAT’S IT!
- Slides: 28