Ionic Bonding An atoms electron configuration explains why
Ionic Bonding An atoms electron configuration explains why an atom will ___________. - All atoms want to be “____” with a complete octet of electrons (__________)

Octet Rule: 1. Atoms want to achieve electron configurations like those of the __________. - They want this because having a full outer energy level makes the atom or ion more ___________. 2. To fulfill the octet rule an atom must have eight electrons in its outer energy or full _____ sublevels.
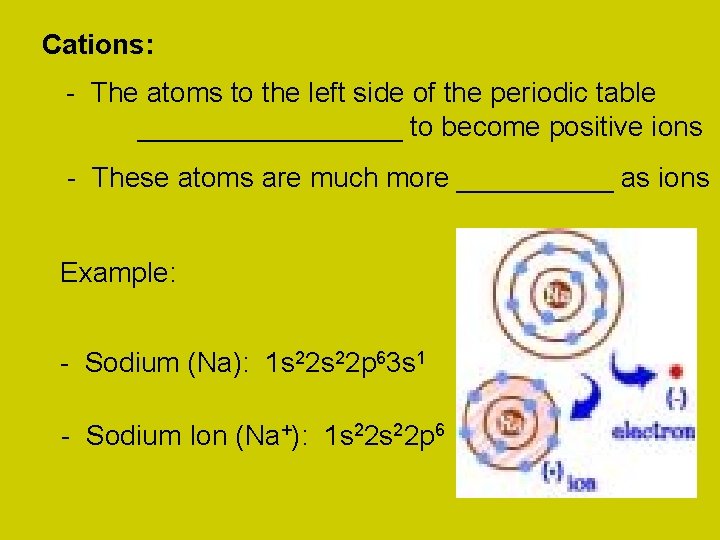
Cations: - The atoms to the left side of the periodic table _________ to become positive ions - These atoms are much more _____ as ions Example: - Sodium (Na): 1 s 22 p 63 s 1 - Sodium Ion (Na+): 1 s 22 p 6
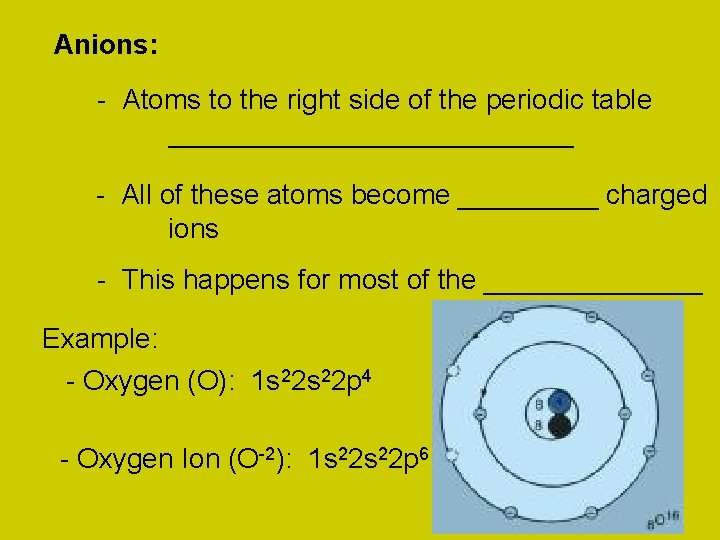
Anions: - Atoms to the right side of the periodic table _____________ - All of these atoms become _____ charged ions - This happens for most of the _______ Example: - Oxygen (O): 1 s 22 p 4 - Oxygen Ion (O-2): 1 s 22 p 6
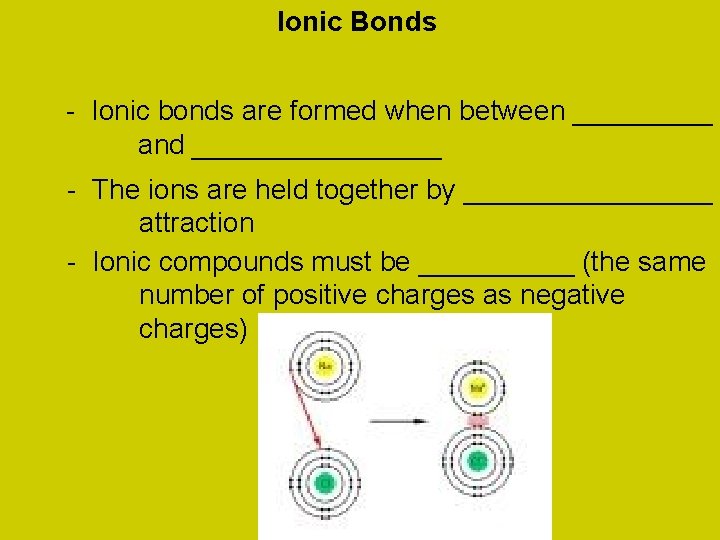
Ionic Bonds - Ionic bonds are formed when between _____ and ________ - The ions are held together by ________ attraction - Ionic compounds must be _____ (the same number of positive charges as negative charges)
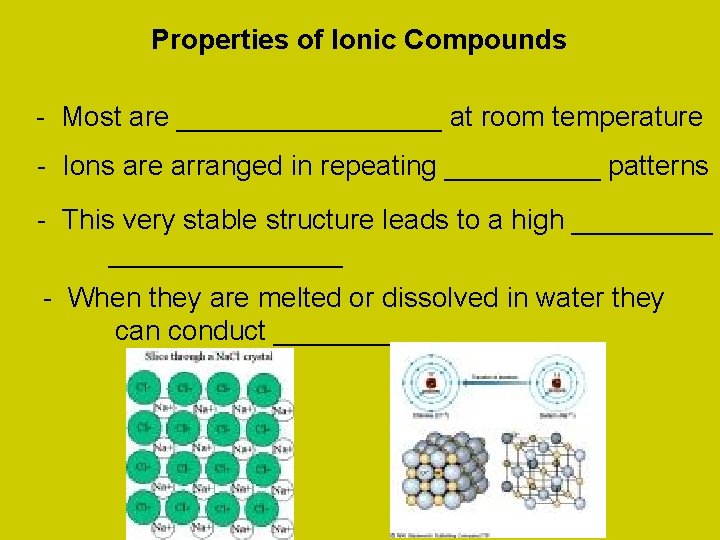
Properties of Ionic Compounds - Most are _________ at room temperature - Ions are arranged in repeating _____ patterns - This very stable structure leads to a high _______________ - When they are melted or dissolved in water they can conduct __________

Bonding in Metals are: 1. made of ______ packed cations 2. cations are _______ by a sea of electrons Metallic Bonds: metals bond due to the ____ of the positively charged cations to the free valence electrons

The Chemical Properties of Metals: - Metals conduct ______ do to the free floating electrons that they contain. - Metals also conduct ____ well because of the free electrons that they contain. - The heat makes the electrons move and the electrons ______ the cations to spread the heat energy. http: //www. gcse. com/energy/conduction 2. htm
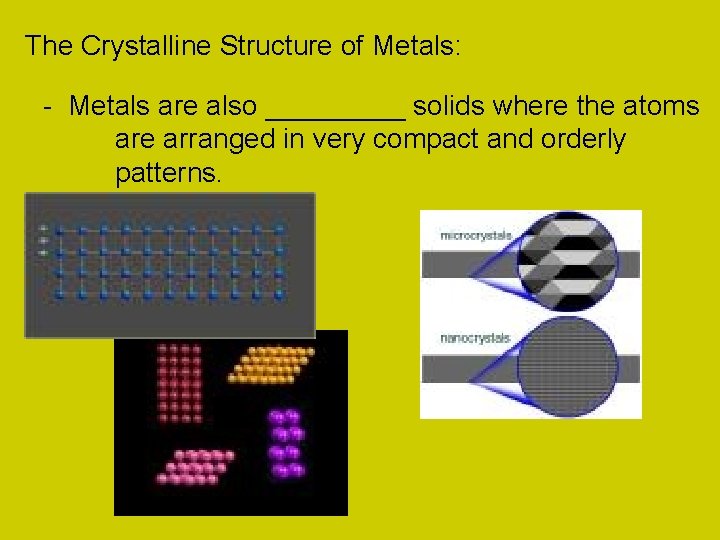
The Crystalline Structure of Metals: - Metals are also _____ solids where the atoms are arranged in very compact and orderly patterns.
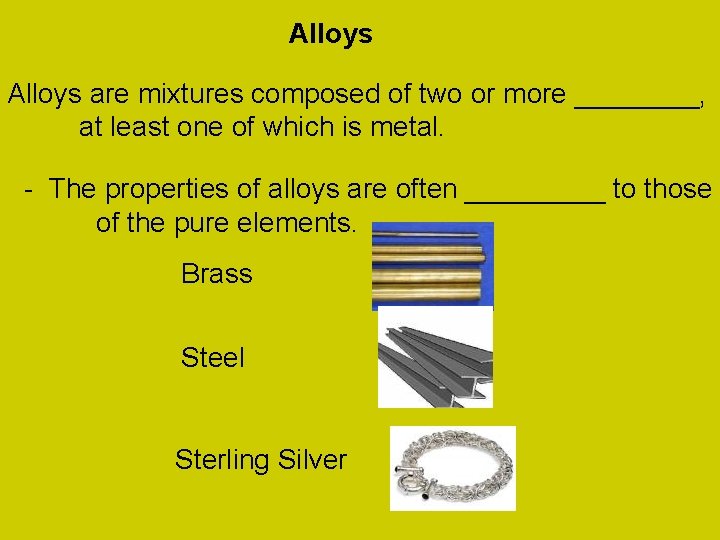
Alloys are mixtures composed of two or more ____, at least one of which is metal. - The properties of alloys are often _____ to those of the pure elements. Brass Steel Sterling Silver
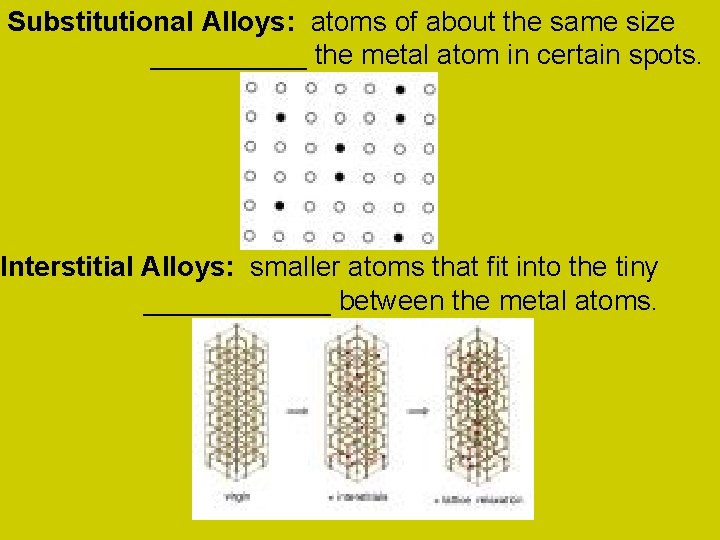
Substitutional Alloys: atoms of about the same size _____ the metal atom in certain spots. Interstitial Alloys: smaller atoms that fit into the tiny ______ between the metal atoms.

Why is water hard? - Hard water contains the ___ of calcium, magnesium, and iron. - Hard water causes scaly deposits ad soap to not lather.
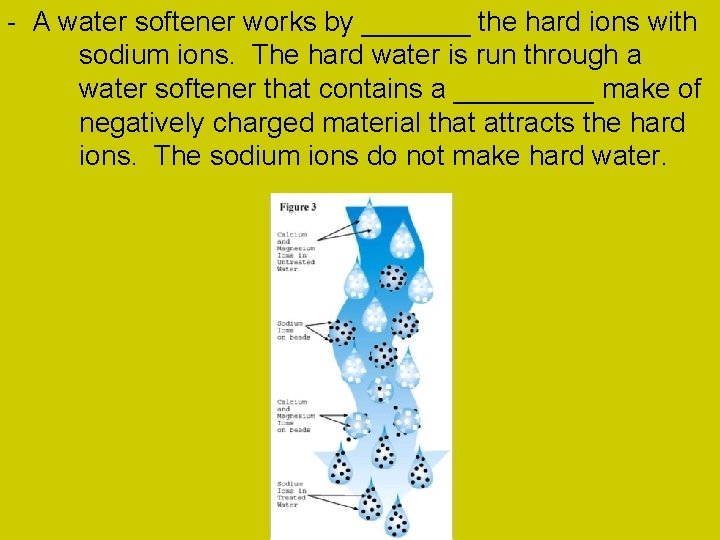
- A water softener works by _______ the hard ions with sodium ions. The hard water is run through a water softener that contains a _____ make of negatively charged material that attracts the hard ions. The sodium ions do not make hard water.
- Slides: 13