Ionic Basis of Resting Membrane and Action Potentials
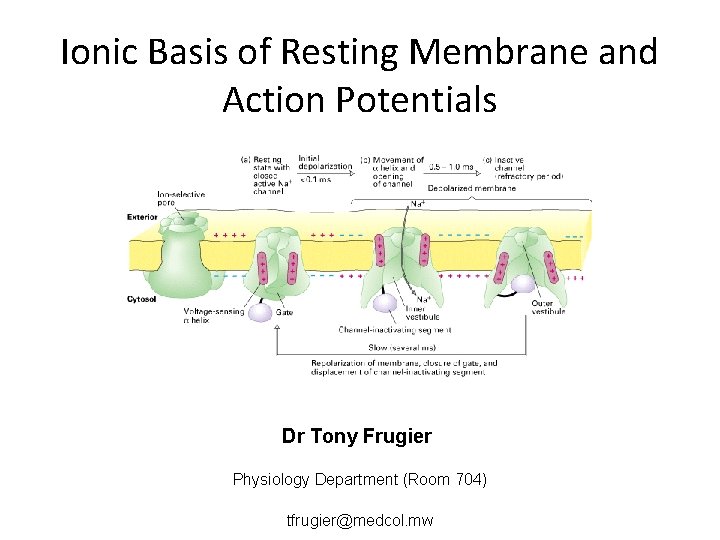
Ionic Basis of Resting Membrane and Action Potentials Dr Tony Frugier Physiology Department (Room 704) tfrugier@medcol. mw
Learning objectives Describe the resting membrane potential Explain equilibrium potential Describe the phases of the action potential Describe the role of the voltage-gated Na and K channels • Describe the AP refractory period • Describe saltatory conduction • Describe frequency code • •
The Resting Membrane Potential • The action of ion transporters creates substantial transmembrane gradients for most ions. • Measurements of the ion concentrations directly in squid neurons, by Alan Hodgkin and Bernard Katz in 1949, are the basis for stating that there is much more K+ inside the neuron than out, and much more Na+ outside than in. • Similar concentration gradients occur in the neurons of most animals, including humans. • However, because the ionic strength of mammalian blood is lower than that of sea-dwelling animals such as squid, in mammals the concentrations of each ion are several times lower. • These transporter-dependent concentration gradients are the indirect source of the resting neuronal membrane potential and the action potential.
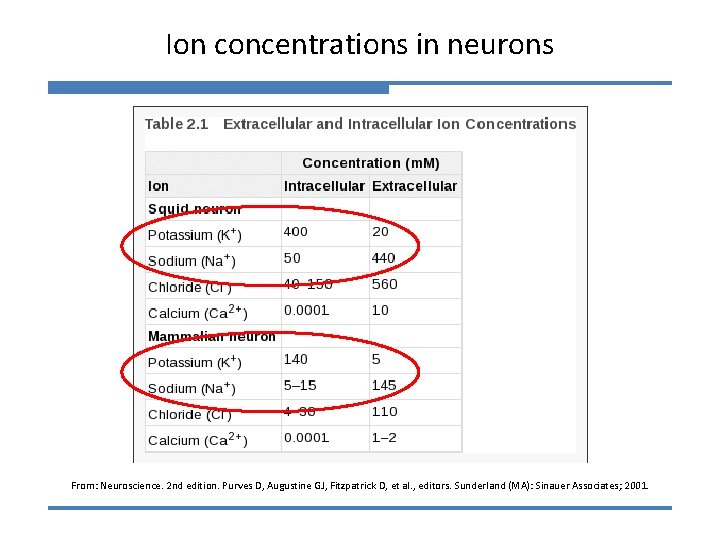
Ion concentrations in neurons From: Neuroscience. 2 nd edition. Purves D, Augustine GJ, Fitzpatrick D, et al. , editors. Sunderland (MA): Sinauer Associates; 2001.
The Resting Membrane Potential • Once the ion concentration gradients across various neuronal membranes are known, the Nernst equation can be used to calculate that the equilibrium potential for K+ (EK) and other major ions. • Since the resting membrane potential (Vrest) of the squid neuron is approximately -65 m. V, K+ is the ion that is closest to being in electrochemical equilibrium when the cell is at rest. • This fact implies that the resting membrane is more permeable to K+ than to the other ions, and that this permeability is the source of resting potentials.
![Electrochemical Equilibrium Linear relationship between transmembrane [K+] gradient and the membrane potential From: Neuroscience. Electrochemical Equilibrium Linear relationship between transmembrane [K+] gradient and the membrane potential From: Neuroscience.](http://slidetodoc.com/presentation_image_h2/a4f7ce5619553e90b044a3ff4877558c/image-6.jpg)
Electrochemical Equilibrium Linear relationship between transmembrane [K+] gradient and the membrane potential From: Neuroscience. 2 nd edition. Purves D, Augustine GJ, Fitzpatrick D, et al. , editors. Sunderland (MA): Sinauer Associates; 2001.
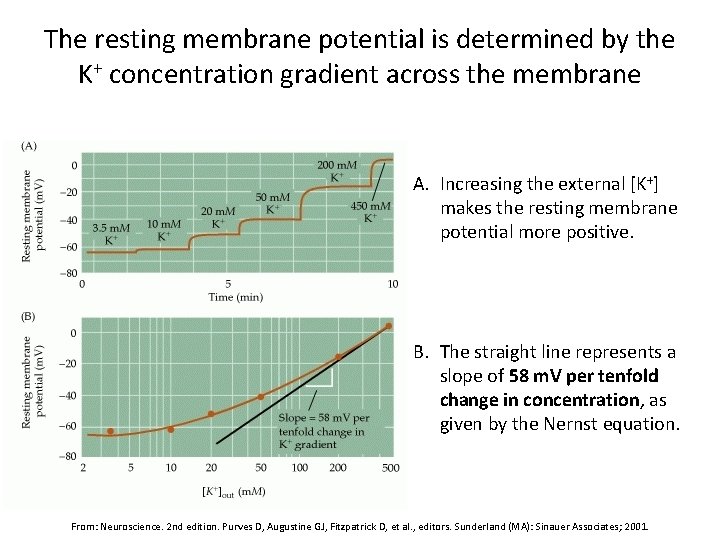
The resting membrane potential is determined by the K+ concentration gradient across the membrane A. Increasing the external [K+] makes the resting membrane potential more positive. B. The straight line represents a slope of 58 m. V per tenfold change in concentration, as given by the Nernst equation. From: Neuroscience. 2 nd edition. Purves D, Augustine GJ, Fitzpatrick D, et al. , editors. Sunderland (MA): Sinauer Associates; 2001.
![The Resting Membrane Potential • The resting membrane potential changes when the external [K+] The Resting Membrane Potential • The resting membrane potential changes when the external [K+]](http://slidetodoc.com/presentation_image_h2/a4f7ce5619553e90b044a3ff4877558c/image-8.jpg)
The Resting Membrane Potential • The resting membrane potential changes when the external [K+] is modified, becoming less negative as external [K+] is raised. • When the external [K+] is raised high enough to equal the [K+] inside the neuron, thus making the K+ equilibrium potential 0 m. V, the resting membrane potential is also approximately 0 m. V. • The resting membrane potential varies as predicted with the logarithm of the [K+] but other ions, such as Cl- and Na+, are also slightly permeable, and thus influence the resting potential to a small degree. • The contribution of these other ions is particularly evident at low external K+ levels, again as predicted by the Goldman equation. • In general, manipulation of the external concentrations of these other ions has only a small effect, emphasizing that K+ permeability is the primary source of the resting membrane potential.
The Resting Membrane Potential: summary The inside-negative resting potential arises because: Ø The membrane of the resting neuron is more permeable to K+ than to any of the other ions present, and Ø There is more K+ inside the neuron than outside. The selective permeability to K+ is caused by K+-permeable membrane channels that are open in resting neurons, and the large [K+] gradient is produced by membrane transporters that selectively accumulate K+ within neurons.
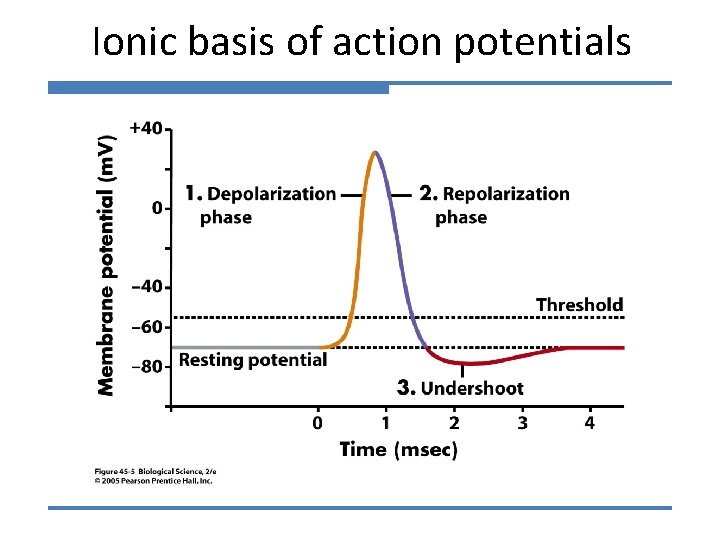
Ionic basis of action potentials
Definition An action potential is a momentary reversal in electrical potential across a plasma membrane (neuron or muscle fiber) that occurs when a cell has been activated by a stimulus
![• In a resting state, the membrane is polarized and the [K+] is • In a resting state, the membrane is polarized and the [K+] is](http://slidetodoc.com/presentation_image_h2/a4f7ce5619553e90b044a3ff4877558c/image-12.jpg)
• In a resting state, the membrane is polarized and the [K+] is higher inside the neuron and the [Na+] is higher outside the neuron
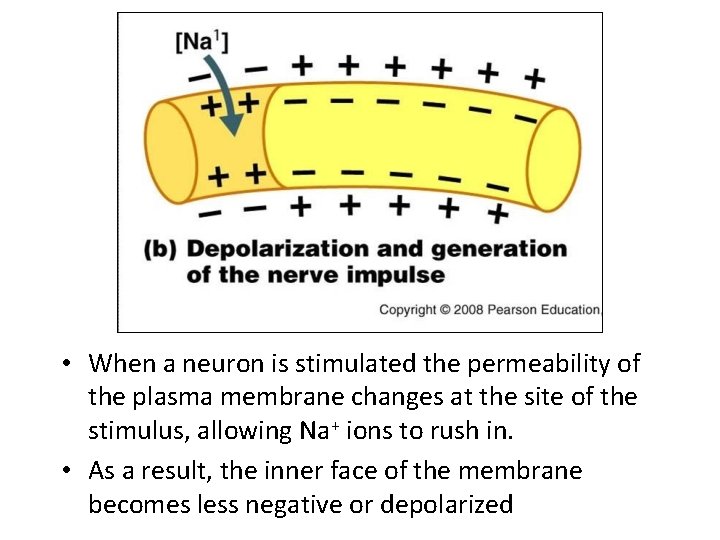
• When a neuron is stimulated the permeability of the plasma membrane changes at the site of the stimulus, allowing Na+ ions to rush in. • As a result, the inner face of the membrane becomes less negative or depolarized
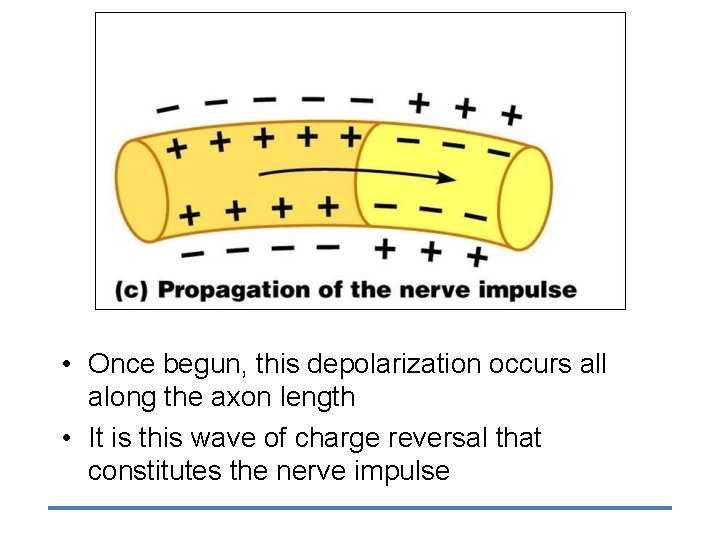
• Once begun, this depolarization occurs all along the axon length • It is this wave of charge reversal that constitutes the nerve impulse
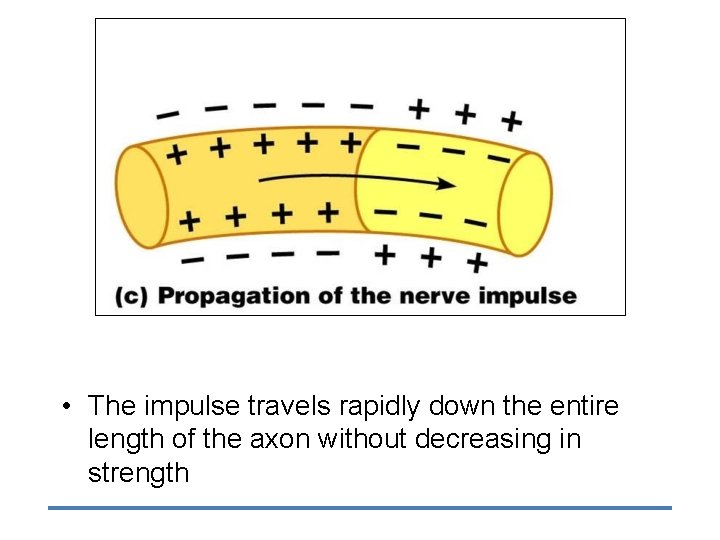
• The impulse travels rapidly down the entire length of the axon without decreasing in strength
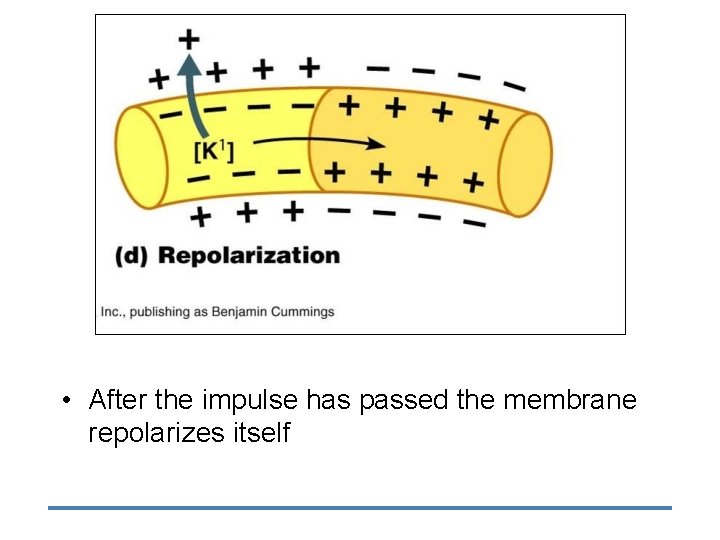
• After the impulse has passed the membrane repolarizes itself
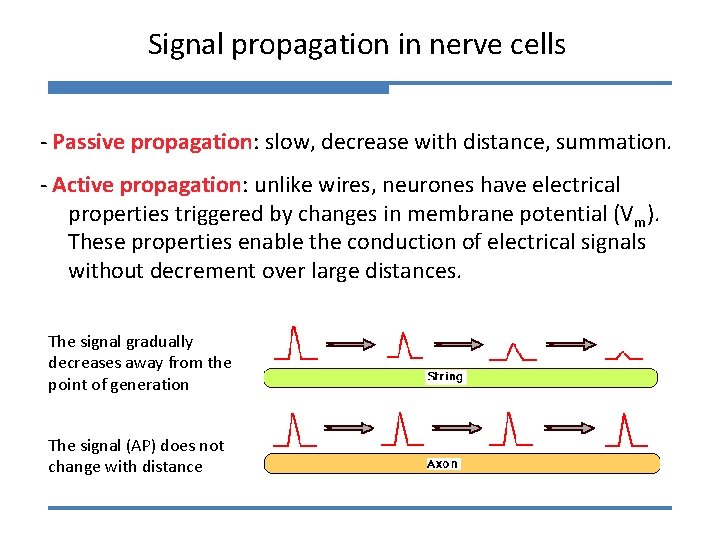
Signal propagation in nerve cells - Passive propagation: slow, decrease with distance, summation. - Active propagation: unlike wires, neurones have electrical properties triggered by changes in membrane potential (Vm). These properties enable the conduction of electrical signals without decrement over large distances. The signal gradually decreases away from the point of generation The signal (AP) does not change with distance
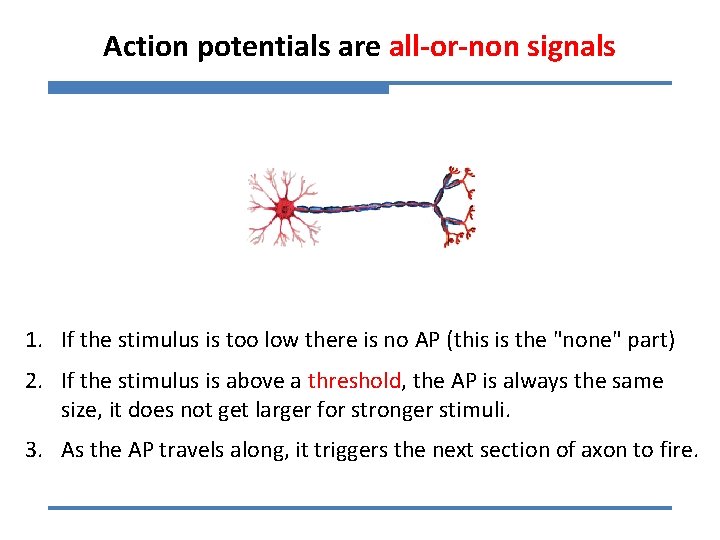
Action potentials are all-or-non signals 1. If the stimulus is too low there is no AP (this is the "none" part) 2. If the stimulus is above a threshold, the AP is always the same size, it does not get larger for stronger stimuli. 3. As the AP travels along, it triggers the next section of axon to fire.
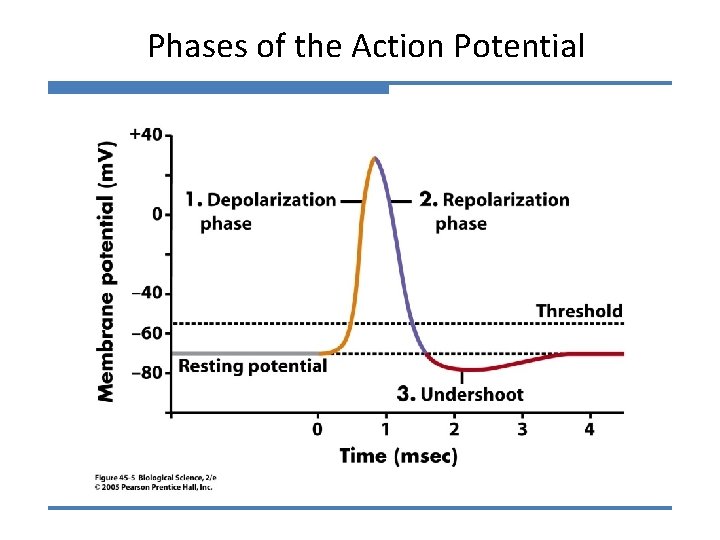
Phases of the Action Potential
Hodgkin and Huxley, in the 1950’s, developed a model to describe how a cell produces an action potential upon stimulation through interactions of voltage-gated Na+ and K+ channels. The Nobel Prize in Physiology or Medicine 1963 "for their discoveries concerning the ionic mechanisms involved in excitation and inhibition in the peripheral and central portions of the nerve cell membrane" Sir John Carew Eccles Alan Lloyd Hodgkin Andrew Fielding Huxley Australia Great Britain Australian National University Canberra, Australia Cambridge University Cambridge, Great Britain London University London, Great Britain 1903 - 1997 1914 - 1998 1917 - 2012
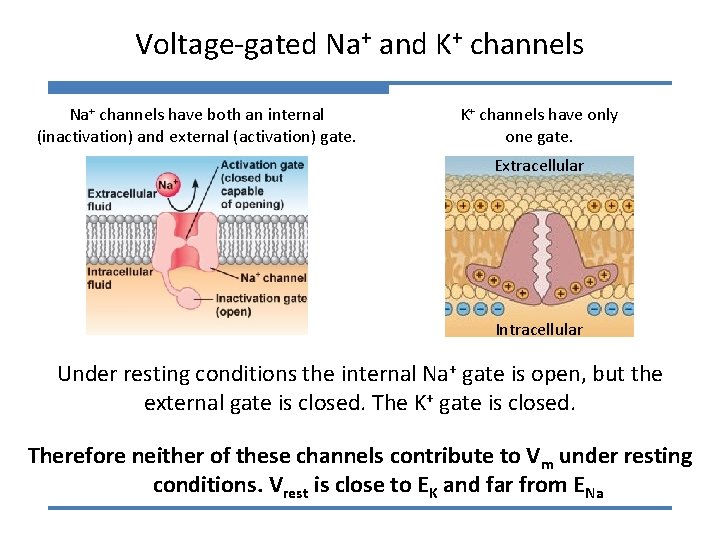
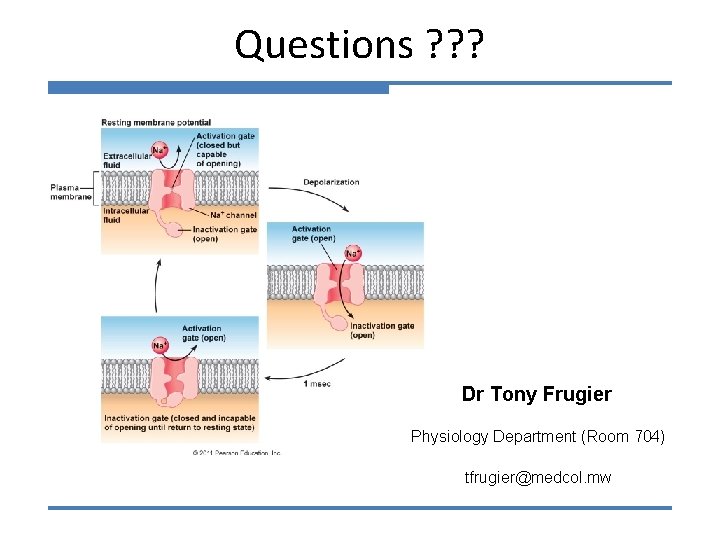
Voltage-gated Na+ and K+ channels Na+ channels have both an internal (inactivation) and external (activation) gate. K+ channels have only one gate. Extracellular Intracellular Under resting conditions the internal Na+ gate is open, but the external gate is closed. The K+ gate is closed. Therefore neither of these channels contribute to Vm under resting conditions. Vrest is close to EK and far from ENa
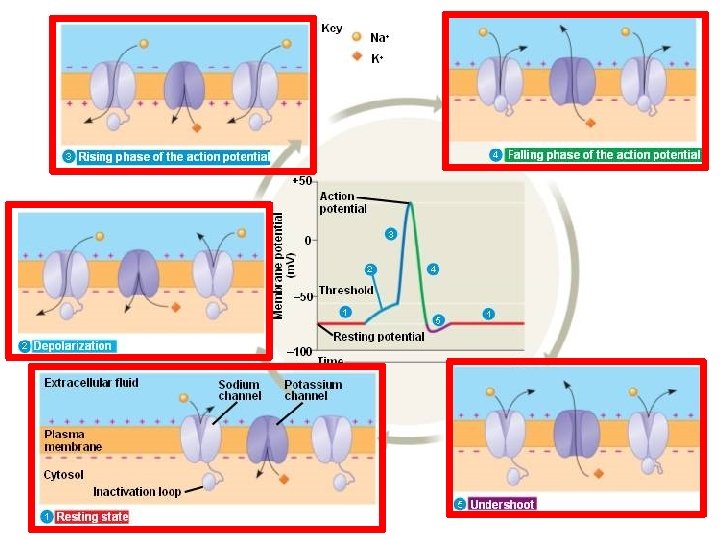
Phases of the Action Potential 1) Depolarization and rising phase: External gates of the Na+ channel start opening: Vm decreases to threshold. Na+ ions flow inside the axon, causing a further decrease in Vm , which will result in more Na+ gates opening. This sequence of events represents a positive feedback mechanism that leads to an ‘explosive’ opening of more and more Na+ channels. 2) Peak of the AP: As a result of the positive feedback during the rising phase, the increased Na+ permeability causes Vm to rapidly move towards ENa. The action potential overshoots and reaches its peak value.
Phases of the Action Potential 3) Falling phase of the AP: The internal Na+ gates inactivate because of the strong depolarisation. This rapid decrease in PNa prevents further depolarisation. Vm starts to repolarise, returning towards EK. The closing of Na+ internal gates is what is responsible for the: ABSOLUTE REFRACTORY PERIOD UNTIL THESE GATES BEGIN TO OPEN THE MEMBRANE WILL NOT GENERATE A SECOND ACTION POTENTIAL
Phases of the Action Potential 3) Falling phase of the AP: The internal Na+ gates inactivate because of the strong depolarisation. This rapid decrease in PNa prevents further depolarisation. Vm starts to repolarise, returning towards EK. The closing of Na+ internal gates is what is responsible for the: ABSOLUTE REFRACTORY PERIOD 4) Role of K+ channels in the repolarisation phase: - delayed K+ channels open (called delayed rectifier) - open after about 1 -2 msec of threshold depolarisation - K+ flows out of the cell and speeds the repolarisation process
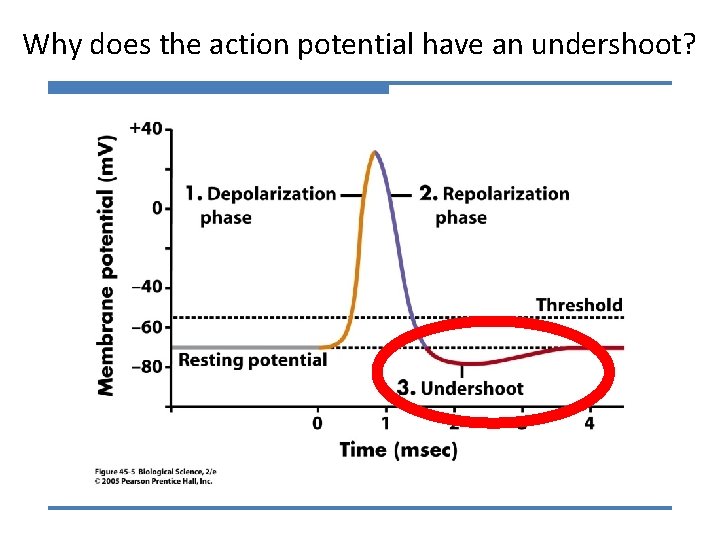
Why does the action potential have an undershoot?
The undershoot of the action potential • Delayed K+ channels open (delayed rectifier). PK higher than at rest and membrane more negative on inside (voltage moves towards EK). • Hyperpolarisation of membrane causes K+ voltage-gated channels to close. • Membrane settles back to rest. • At the same time, the internal Na+ gates re-open, and the membrane is ready to generate another action potential. Ø These events are responsible for the: RELATIVE REFRACTORY PERIOD
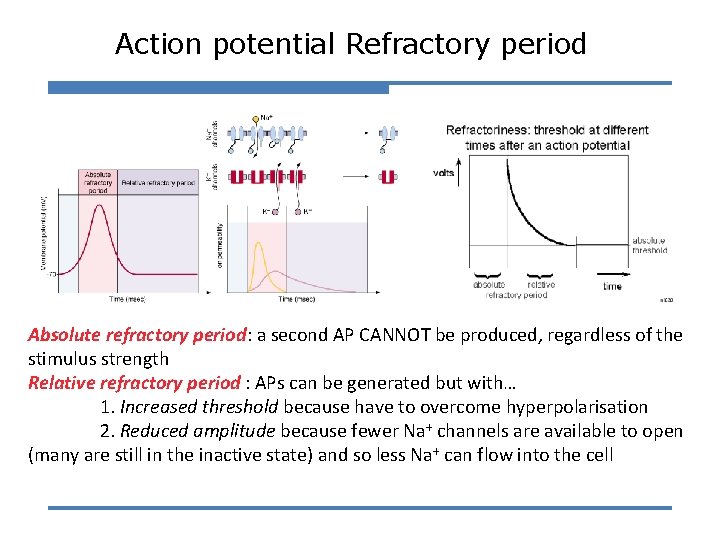
Action potential Refractory period Absolute refractory period: a second AP CANNOT be produced, regardless of the stimulus strength Relative refractory period : APs can be generated but with… 1. Increased threshold because have to overcome hyperpolarisation 2. Reduced amplitude because fewer Na+ channels are available to open (many are still in the inactive state) and so less Na+ can flow into the cell
The action potential always propagates forward Why doesn’t it backfire?
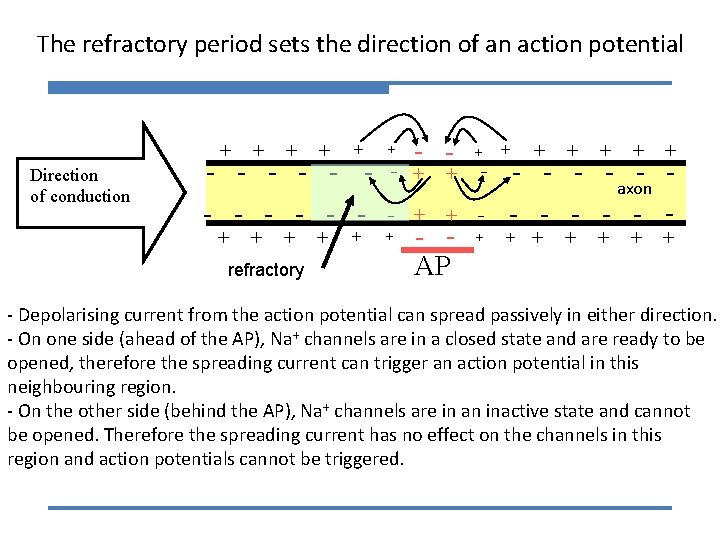
The refractory period sets the direction of an action potential + Direction of conduction + + - - - - - + + + refractory + + + - - + + + - + + - - - - axon - - - + + + - AP + - Depolarising current from the action potential can spread passively in either direction. - On one side (ahead of the AP), Na+ channels are in a closed state and are ready to be opened, therefore the spreading current can trigger an action potential in this neighbouring region. - On the other side (behind the AP), Na+ channels are in an inactive state and cannot be opened. Therefore the spreading current has no effect on the channels in this region and action potentials cannot be triggered.
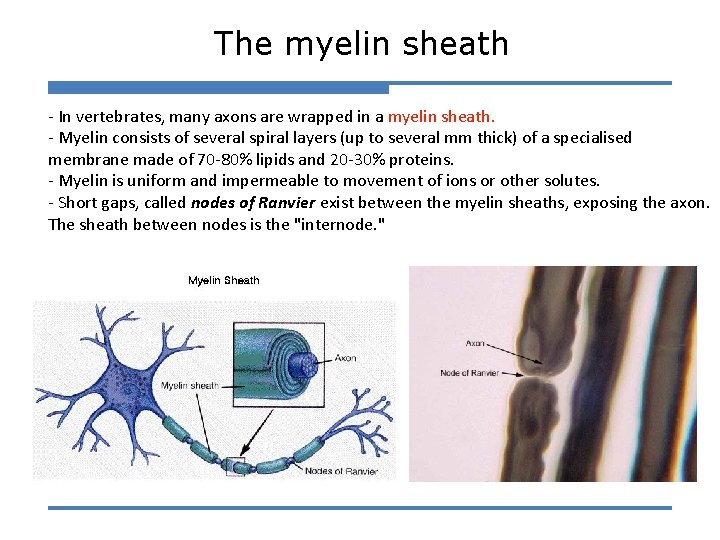
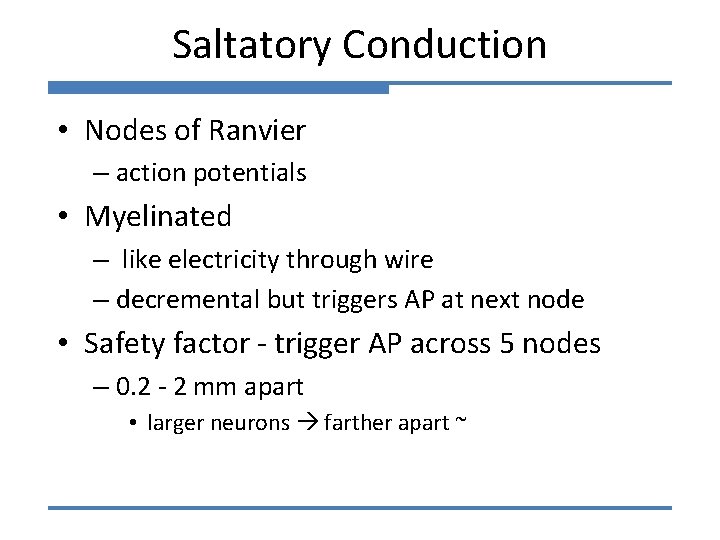
The myelin sheath - In vertebrates, many axons are wrapped in a myelin sheath. - Myelin consists of several spiral layers (up to several mm thick) of a specialised membrane made of 70 -80% lipids and 20 -30% proteins. - Myelin is uniform and impermeable to movement of ions or other solutes. - Short gaps, called nodes of Ranvier exist between the myelin sheaths, exposing the axon. The sheath between nodes is the "internode. "
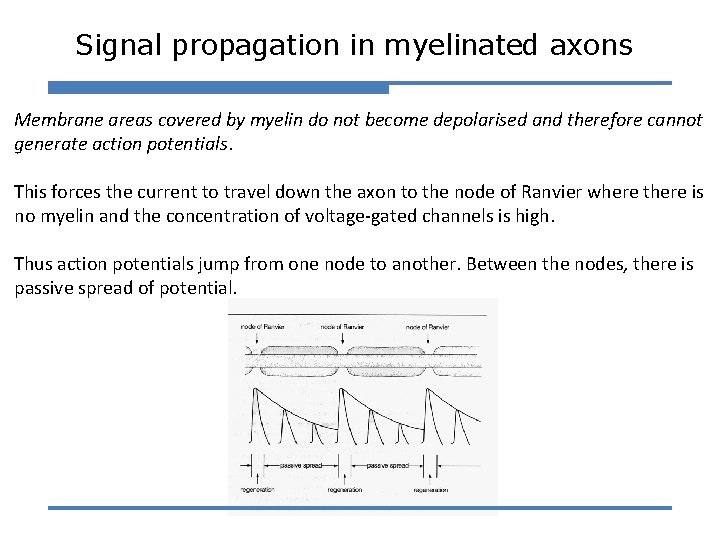
Signal propagation in myelinated axons Membrane areas covered by myelin do not become depolarised and therefore cannot generate action potentials. This forces the current to travel down the axon to the node of Ranvier where there is no myelin and the concentration of voltage-gated channels is high. Thus action potentials jump from one node to another. Between the nodes, there is passive spread of potential.
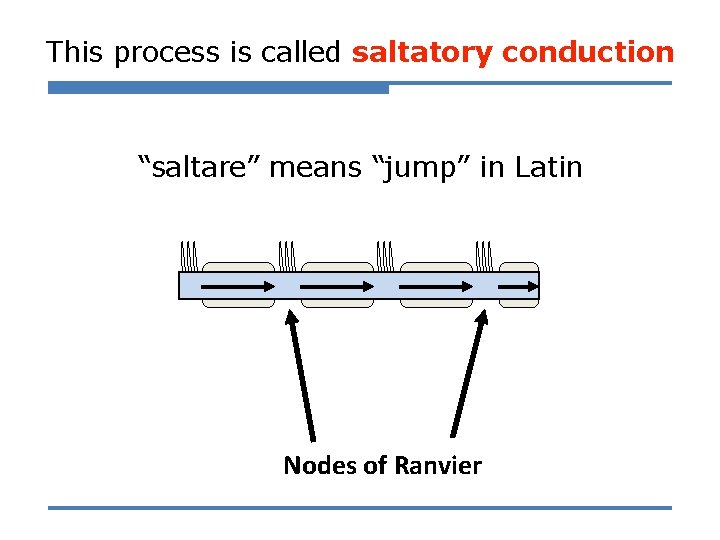
This process is called saltatory conduction “saltare” means “jump” in Latin Nodes of Ranvier
Functional consequences of saltatory conduction in myelinated axons Saltatory conduction significantly increases the conduction velocity Small non-myelinated axons: conduction velocity is about 0. 25 m/sec Large myelinated axons: conduction velocity can reach 120 m/sec
Saltatory Conduction • Myelinated neurons – oligodendroglia & Schwann cells • Transmit long distances – APs relatively slow, regenerates – EPSPs - fast, decremental • Saltatory: combines both types of current – speed without loss of signal ~
Saltatory Conduction • Nodes of Ranvier – action potentials • Myelinated – like electricity through wire – decremental but triggers AP at next node • Safety factor - trigger AP across 5 nodes – 0. 2 - 2 mm apart • larger neurons farther apart ~
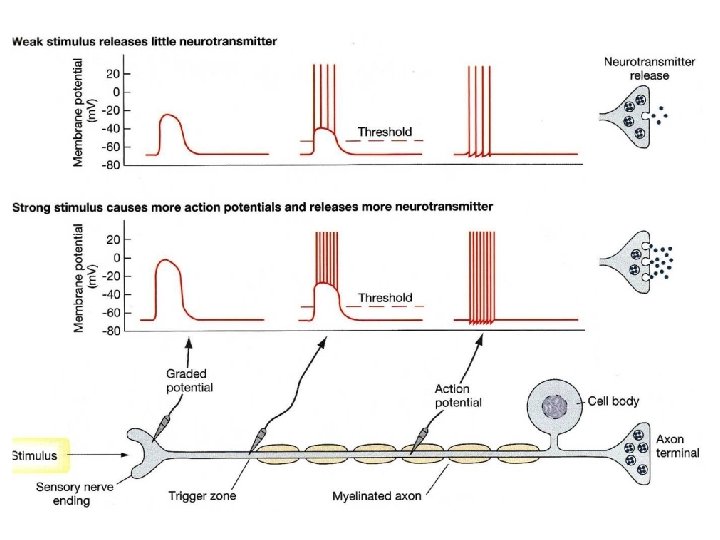
Frequency Code • Pattern = Intensity of stimulus – frequency of APs • Place = type of stimulus – Visual, auditory, pain, etc. – Brain area that receives signal – Doctrine of Specific Nerve Energies ~
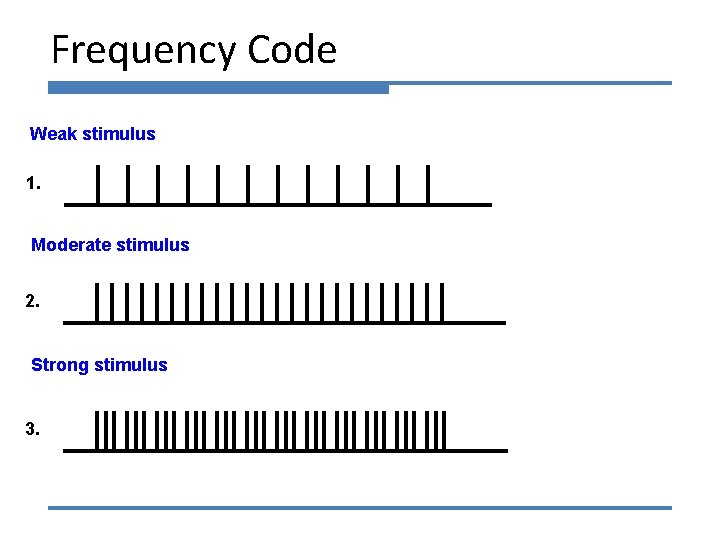
Frequency Code Weak stimulus 1. Moderate stimulus 2. Strong stimulus 3.
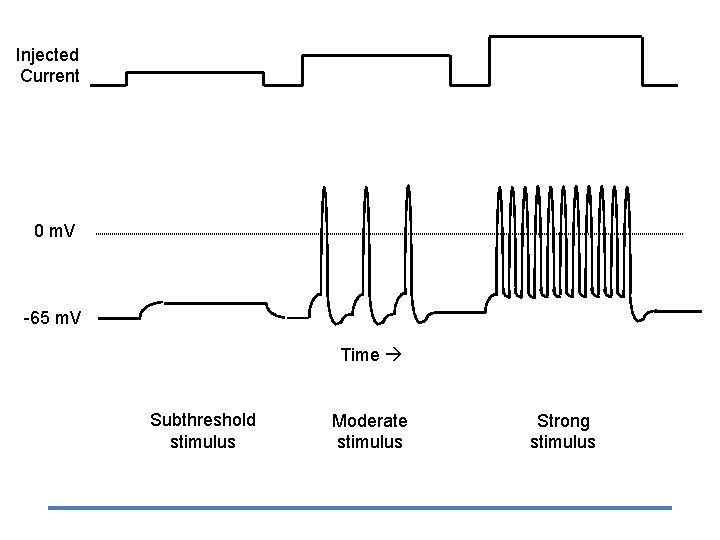
Injected Current 0 m. V -65 m. V Time Subthreshold stimulus Moderate stimulus Strong stimulus
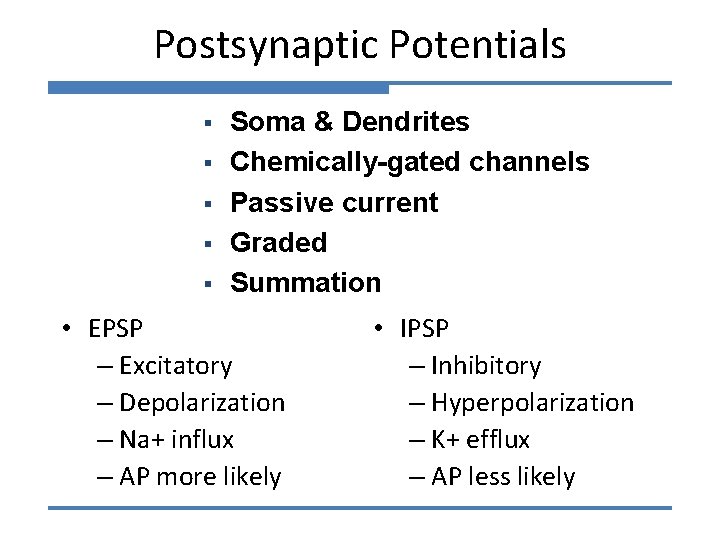
Postsynaptic Potentials § § § Soma & Dendrites Chemically-gated channels Passive current Graded Summation • EPSP – Excitatory – Depolarization – Na+ influx – AP more likely • IPSP – Inhibitory – Hyperpolarization – K+ efflux – AP less likely
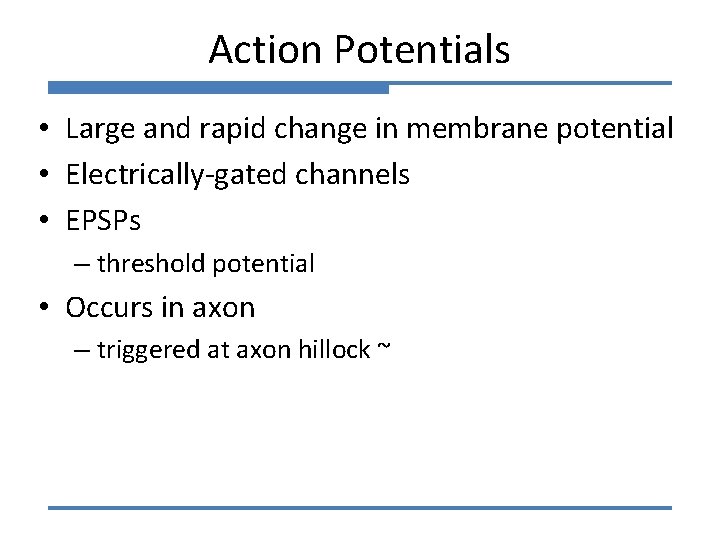
Action Potentials • Large and rapid change in membrane potential • Electrically-gated channels • EPSPs – threshold potential • Occurs in axon – triggered at axon hillock ~
AP Characteristics • • • Voltage-gated channels All or none Slow Non-decremental Self Propagated – regenerated ~
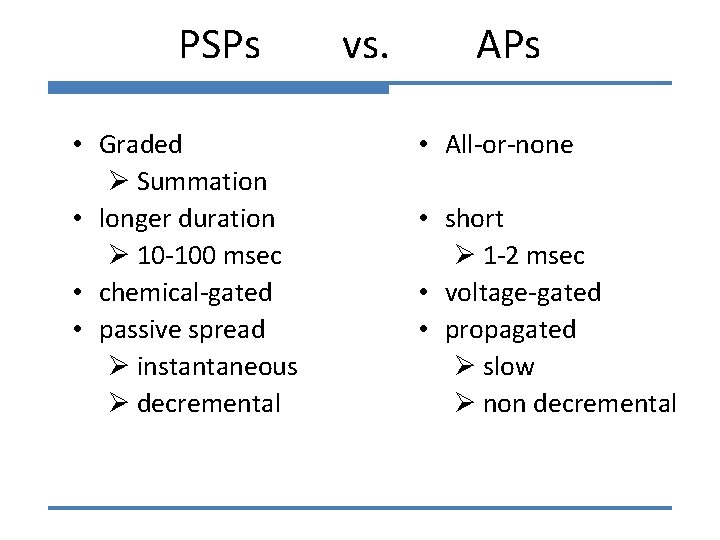
PSPs • Graded Ø Summation • longer duration Ø 10 -100 msec • chemical-gated • passive spread Ø instantaneous Ø decremental vs. APs • All-or-none • short Ø 1 -2 msec • voltage-gated • propagated Ø slow Ø non decremental
Questions ? ? ? Dr Tony Frugier Physiology Department (Room 704) tfrugier@medcol. mw
- Slides: 46