Ionic and Molecular Compounds Chemistry 100 What are














- Slides: 38

Ionic and Molecular Compounds Chemistry 100

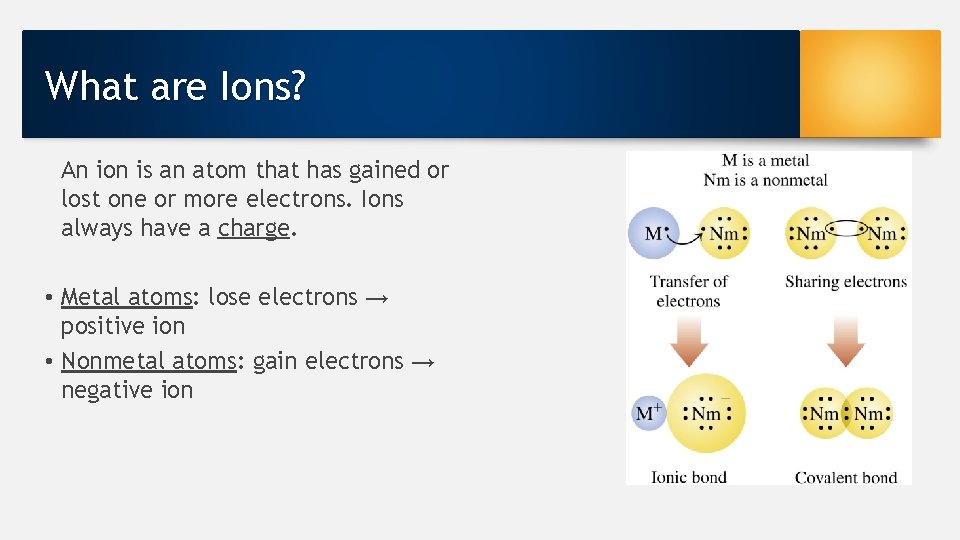
What are Ions? An ion is an atom that has gained or lost one or more electrons. Ions always have a charge. • Metal atoms: lose electrons → positive ion • Nonmetal atoms: gain electrons → negative ion

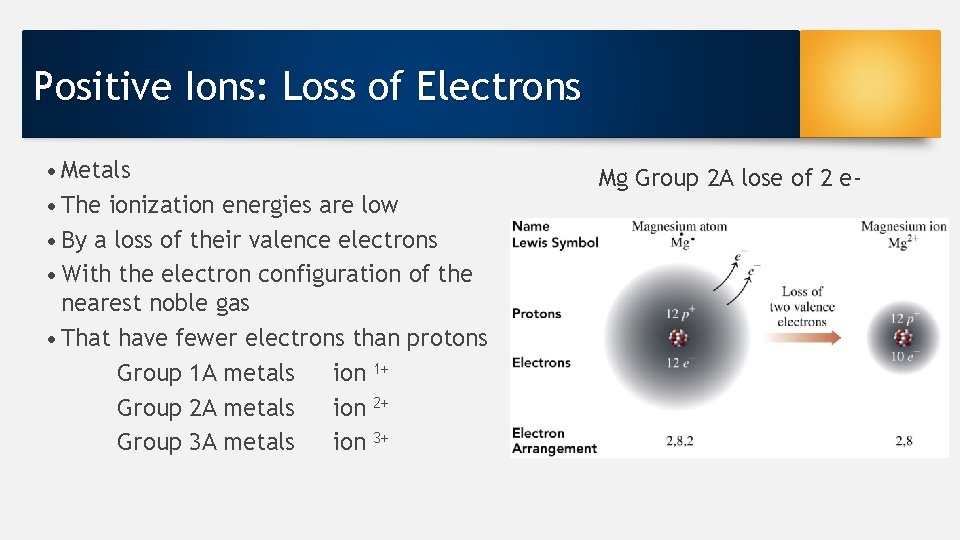
Positive Ions: Loss of Electrons • Metals • The ionization energies are low • By a loss of their valence electrons • With the electron configuration of the nearest noble gas • That have fewer electrons than protons Group 1 A metals ion 1+ Group 2 A metals ion 2+ Group 3 A metals ion 3+ Mg Group 2 A lose of 2 e-

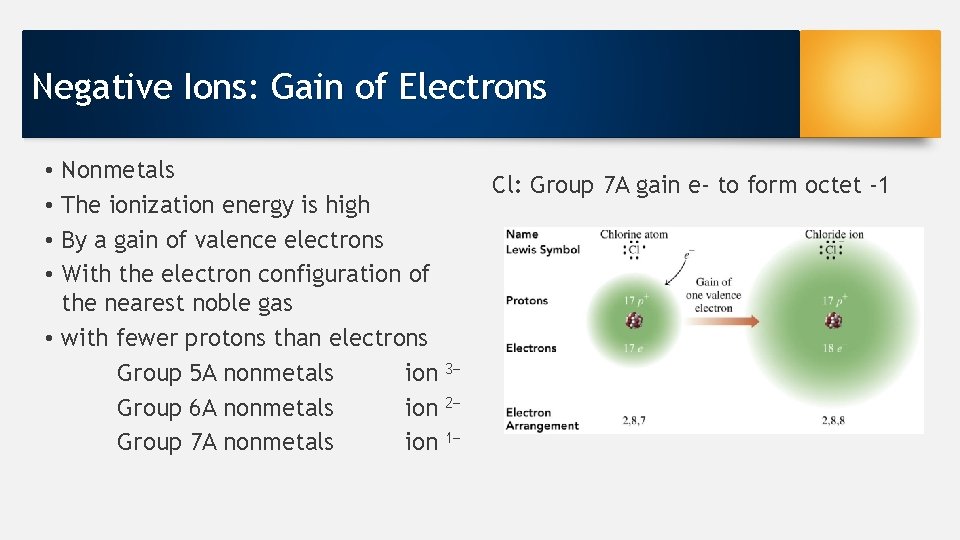
Negative Ions: Gain of Electrons Nonmetals The ionization energy is high By a gain of valence electrons With the electron configuration of the nearest noble gas • with fewer protons than electrons Group 5 A nonmetals ion Group 6 A nonmetals ion Group 7 A nonmetals ion • • Cl: Group 7 A gain e- to form octet -1 3− 2− 1−

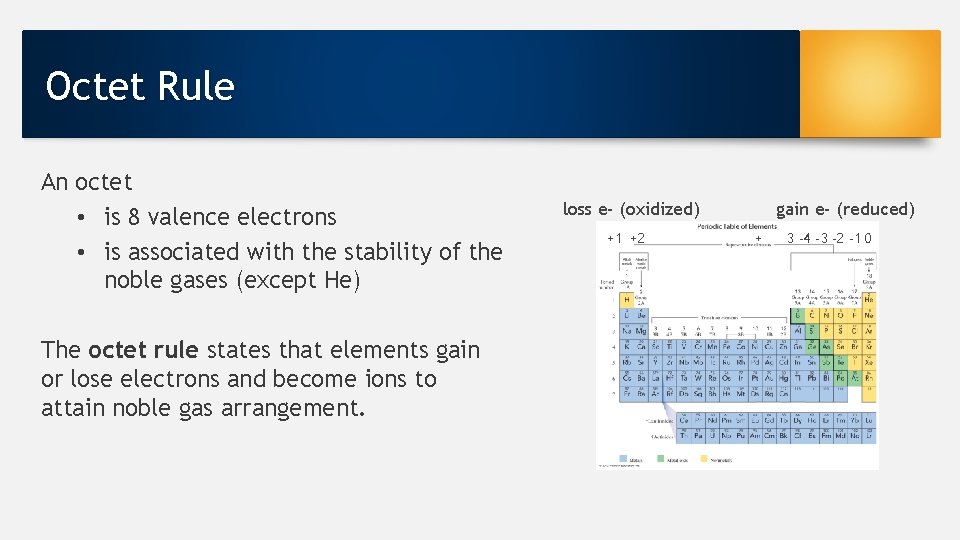
Octet Rule An octet • is 8 valence electrons • is associated with the stability of the noble gases (except He) The octet rule states that elements gain or lose electrons and become ions to attain noble gas arrangement. loss e- (oxidized) +1 +2 gain e- (reduced) + 3 -4 -3 -2 -1 0

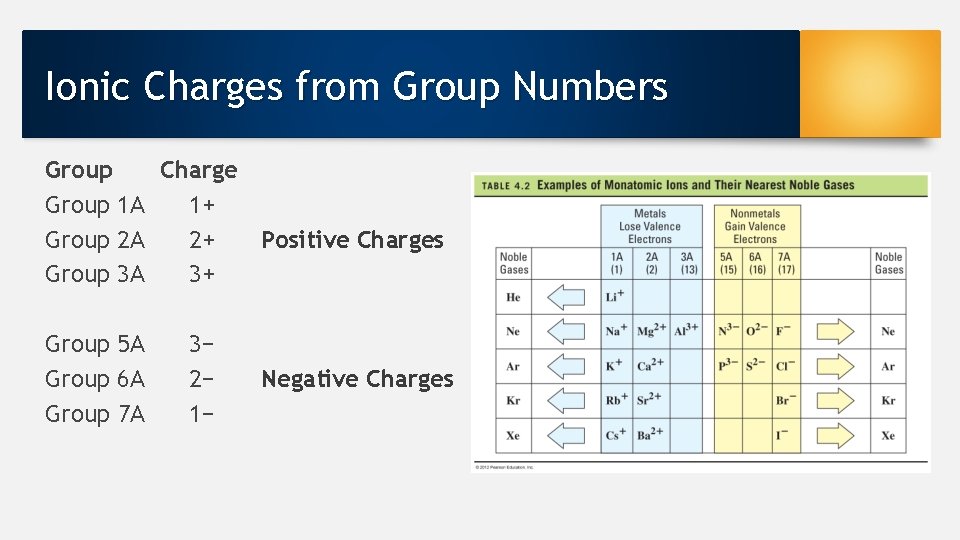
Ionic Charges from Group Numbers Group Charge Group 1 A 1+ Group 2 A 2+ Positive Charges Group 3 A 3+ Group 5 A Group 6 A Group 7 A 3− 2− 1− Negative Charges

Properties of Ionic Compounds • Consist of positive and negative ions • Have attractions called ionic bonds between positively and negatively charged ions • Have high melting points • Are solid at room temperature • Total positive charge = Total negative charge o sum of the ionic charges is always zero - neutral • The symbol of the metal is written first, followed by the symbol of the nonmetal o metal symbol, nonmetal symbol

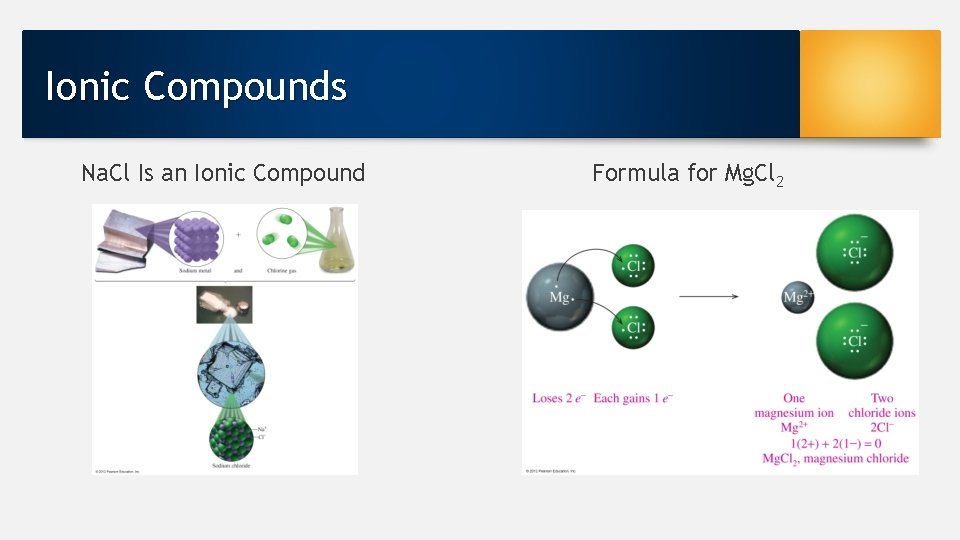
Ionic Compounds Na. Cl Is an Ionic Compound Formula for Mg. Cl 2

Study Check 1 Write the ionic formula of the compound formed with: A. Na+ and O 2− 1) Na. O 2) Na 2 O 3) Na. O 2 B. Al 3+ and Cl− 1) Al. Cl 3 2) Al. Cl 3) Al 3 Cl C. Mg 2+ and N 3− 1) Mg. N 2) Mg 2 N 3 3) Mg 3 N 2

Study Check 1 Answer A. Na+ and O 2− 2) Na 2 O check: 2 Na+ + O 2 - = 2(1+) + 1(2 -) = 0 B. Al 3+ and Cl− 1) Al. Cl 3 check: Al 3+ + 3 Cl- = 1(3+) + 3(1 -) = 0 C. Mg 2+ and N 3− 3) Mg 3 N 2 check: 3 Mg 2+ + 2 N 3 - = 3(2+) + 2(3 -) = 0

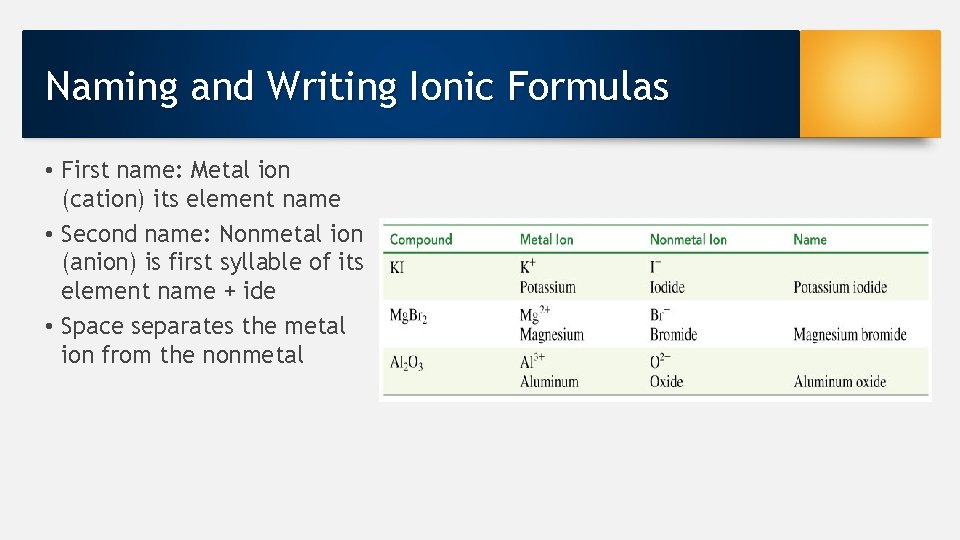
Naming and Writing Ionic Formulas • First name: Metal ion (cation) its element name • Second name: Nonmetal ion (anion) is first syllable of its element name + ide • Space separates the metal ion from the nonmetal

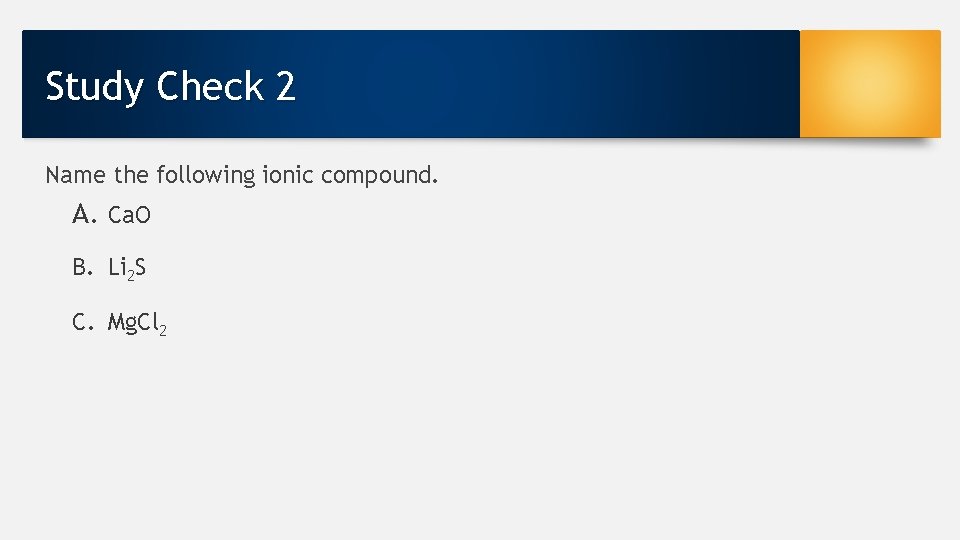
Study Check 2 Name the following ionic compound. A. Ca. O B. Li 2 S C. Mg. Cl 2

Study Check 2 Answers Write the name of the metal first and the name of the nonmetal second with the ending -ide. A. The name of Ca. O is calcium oxide. B. The name of Li 2 S is lithium sulfide. C. The name of Mg. Cl 2 is magnesium chloride.

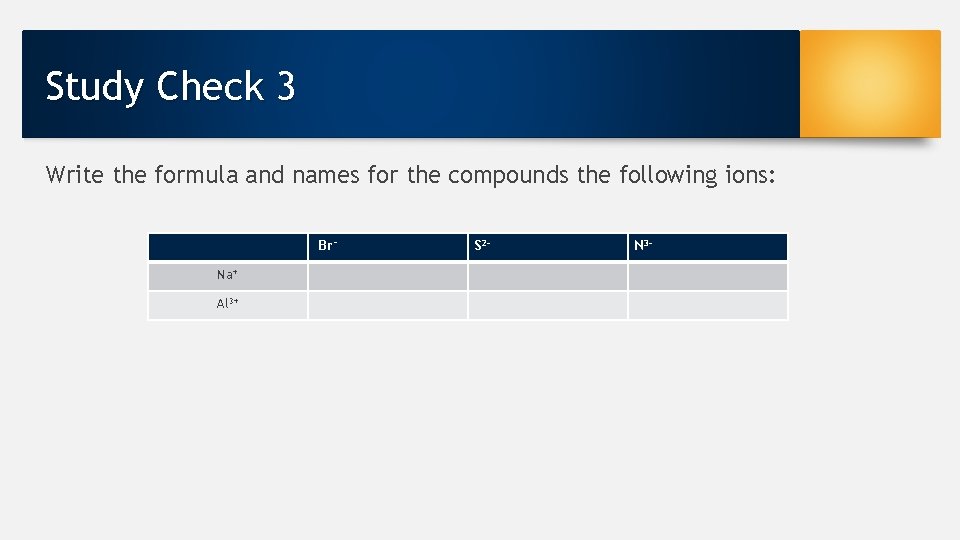
Study Check 3 Write the formula and names for the compounds the following ions: Br− Na+ Al 3+ S 2 - N 3 -

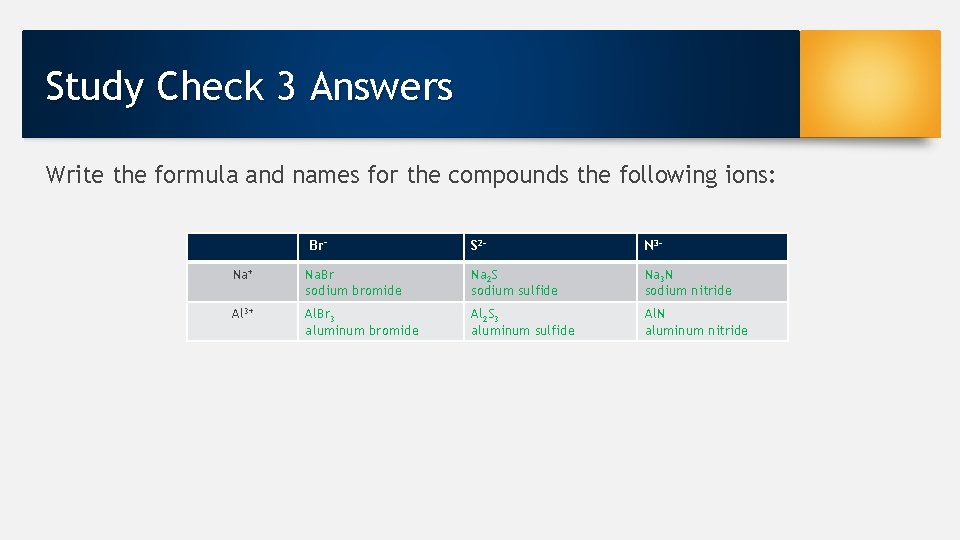
Study Check 3 Answers Write the formula and names for the compounds the following ions: Br− S 2 - N 3 - Na+ Na. Br sodium bromide Na 2 S sodium sulfide Na 3 N sodium nitride Al 3+ Al. Br 3 aluminum bromide Al 2 S 3 aluminum sulfide Al. N aluminum nitride

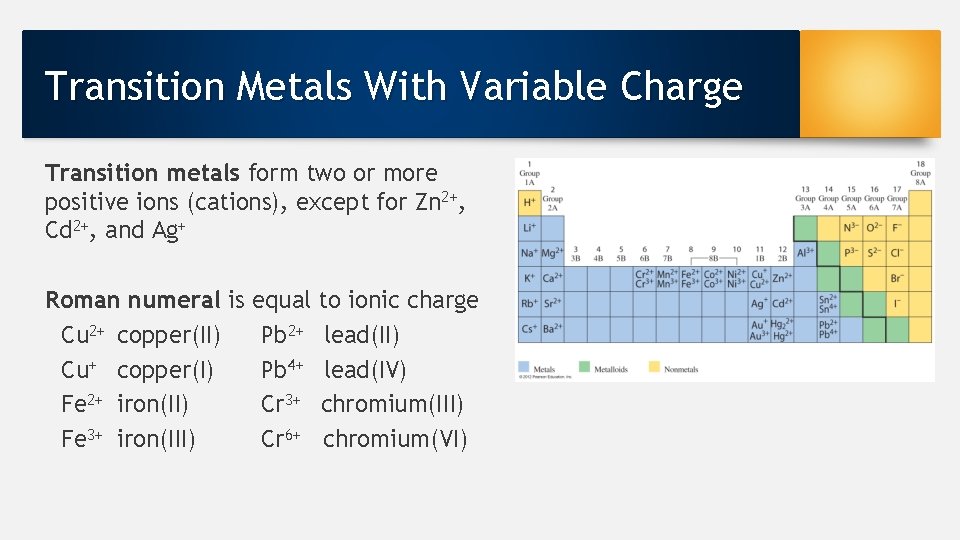
Transition Metals With Variable Charge Transition metals form two or more positive ions (cations), except for Zn 2+, Cd 2+, and Ag+ Roman numeral is Cu 2+ copper(II) Cu+ copper(I) Fe 2+ iron(II) Fe 3+ iron(III) equal Pb 2+ Pb 4+ Cr 3+ Cr 6+ to ionic charge lead(II) lead(IV) chromium(III) chromium(VI)

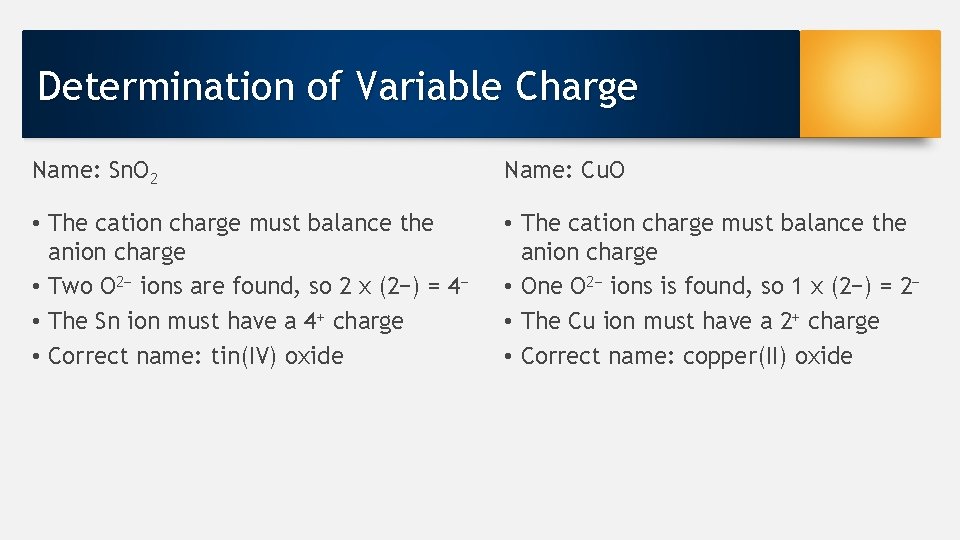
Determination of Variable Charge Name: Sn. O 2 Name: Cu. O • The cation charge must balance the anion charge • Two O 2− ions are found, so 2 x (2−) = 4− • The Sn ion must have a 4+ charge • Correct name: tin(IV) oxide • The cation charge must balance the anion charge • One O 2− ions is found, so 1 x (2−) = 2− • The Cu ion must have a 2+ charge • Correct name: copper(II) oxide

Study Check 4 Name the following ionic compounds: A. B. C. D. Fe. S Zn. O Cu 3 N Ba. Br 2

Study Check 4 Answers Name the following ionic compounds: A. B. C. D. Fe. S Zn. O Cu 3 N Ba. Br 2 iron(II) sulfide zinc oxide copper(I) nitride barium bromide

Study Check 5 Write Chemical Formulas for the following compounds: A. Nickel(II) sulfide B. Zinc chloride C. Iron(III) oxide

Study Check 5 Answers A. Nickel(II) Sulfide Ni 2+ S 2− 1(2+) + 1(2−) = 0 B. Zinc Chloride Zn. Cl 2 Zn 2+ Cl− 1(2+) + 2(1−) = 0 Ni. S C. Iron(III) oxide Fe 2 O 3 The Roman numeral (III) indicates that the charge on the iron is 3+, Fe 3+ O 2− 2(3+) + 3(2−) = 0

Polyatomic Ions • Are a group of atoms that always stick together with a specific ionic charge • Often consist of a nonmetal such as phosphorus, sulfur, carbon, or nitrogen bonded to oxygen • Usually have a 1−, 2−, or 3− charge • Gained 1, 2, or 3 e- to complete octets • NH 4+ only positive charge • Names of most common polyatomic ions end in ate SO 42− sulfate PO 43− phosphate NO 3− nitrate

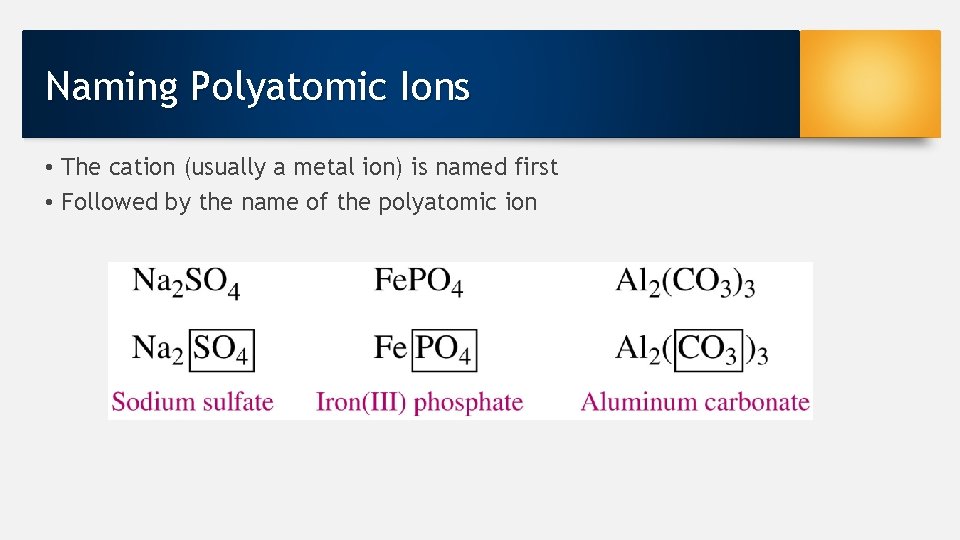
Naming Polyatomic Ions • The cation (usually a metal ion) is named first • Followed by the name of the polyatomic ion

Study Check 6 Name each of the following compounds containing polyatomic ions: A. Mg. SO 3 B. Mg. SO 4 C. Ca(Cl. O 3)2

Study Check 6 Answers Step 1 cation/anion Step 2 Name cation Step 3 Name anion Step 4 Name compound A. Mg. SO 3 Mg 2+ SO 32− magnesium sulfite B. Mg. SO 4 Mg 2+ SO 42− magnesium sulfate C. Ca(Cl. O 3)2 Ca 2+ Cl. O 3− calcium chlorate

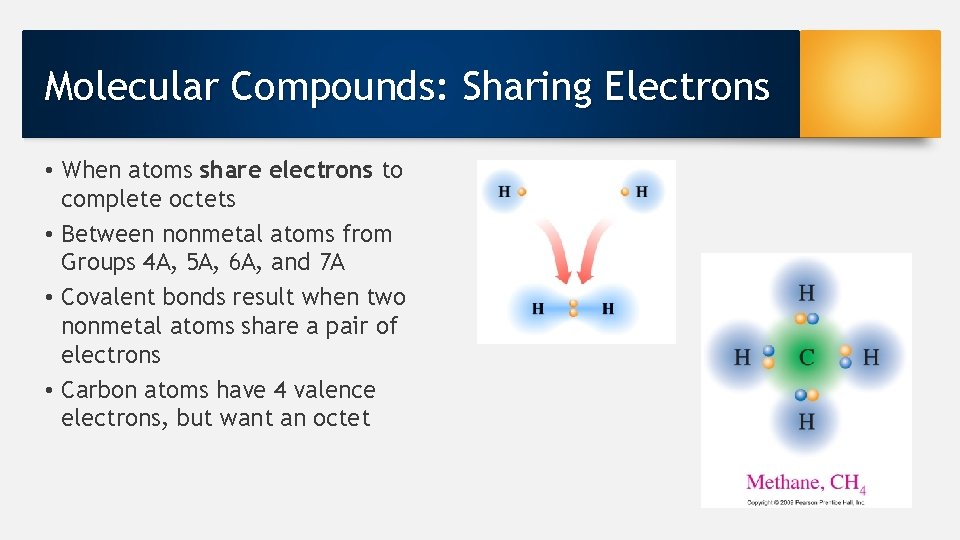
Molecular Compounds: Sharing Electrons • When atoms share electrons to complete octets • Between nonmetal atoms from Groups 4 A, 5 A, 6 A, and 7 A • Covalent bonds result when two nonmetal atoms share a pair of electrons • Carbon atoms have 4 valence electrons, but want an octet

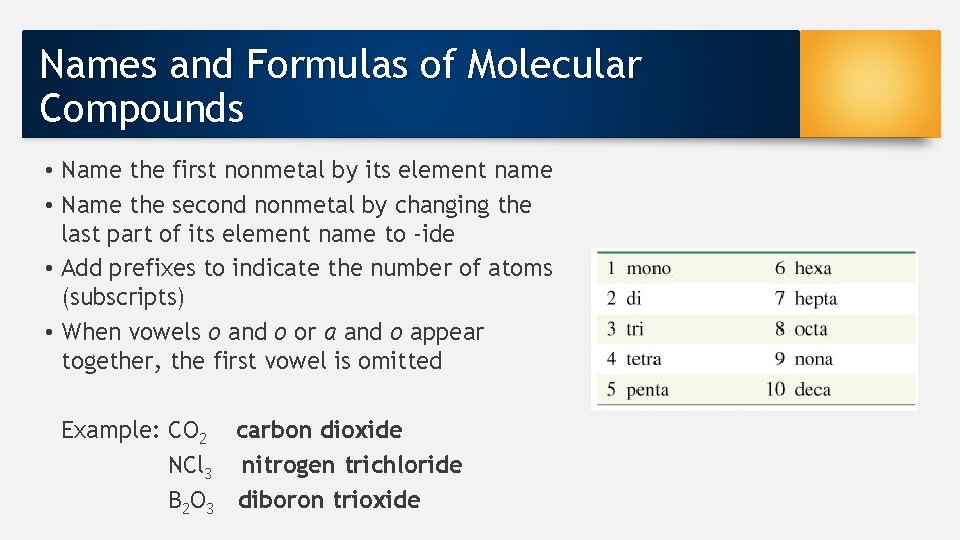
Names and Formulas of Molecular Compounds • Name the first nonmetal by its element name • Name the second nonmetal by changing the last part of its element name to -ide • Add prefixes to indicate the number of atoms (subscripts) • When vowels o and o or a and o appear together, the first vowel is omitted Example: CO 2 carbon dioxide NCl 3 nitrogen trichloride B 2 O 3 diboron trioxide

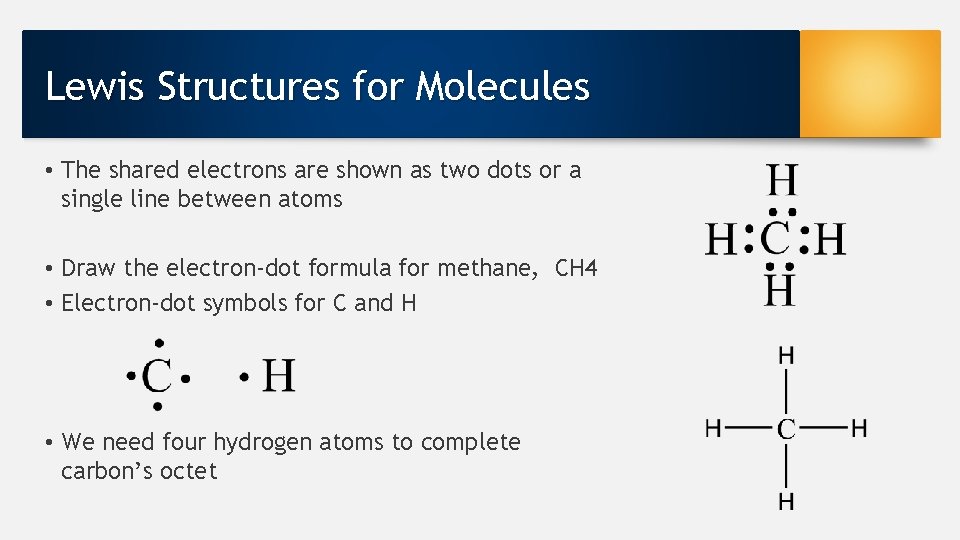
Lewis Structures for Molecules • The shared electrons are shown as two dots or a single line between atoms • Draw the electron-dot formula for methane, CH 4 • Electron-dot symbols for C and H • We need four hydrogen atoms to complete carbon’s octet

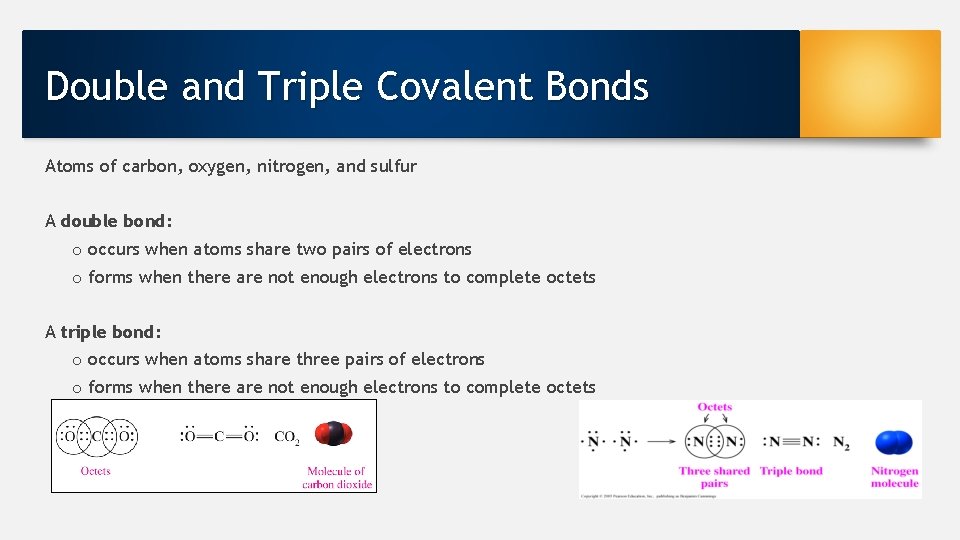
Double and Triple Covalent Bonds Atoms of carbon, oxygen, nitrogen, and sulfur A double bond: o occurs when atoms share two pairs of electrons o forms when there are not enough electrons to complete octets A triple bond: o occurs when atoms share three pairs of electrons o forms when there are not enough electrons to complete octets

Study Check 7 Determine if each compound is ionic (I) or covalent (C ), and write the formula. A. sulfur trioxide B. aluminum carbonate C. dinitrogen tetroxide D. copper(I) sulfate

Study Check 7 Answers Determine if each compound is ionic (I) or covalent (C ), and write the formula. A. sulfur trioxide (C) S, 3 O SO 3 B. aluminum carbonate (I) Al 3+, CO 32− Al 2(CO 3) 3 C. dinitrogen tetroxide (C) 2 N, 4 O N 2 O 4 D. copper(I) sulfate Cu+, SO 42− Cu 2 SO 4 (I)

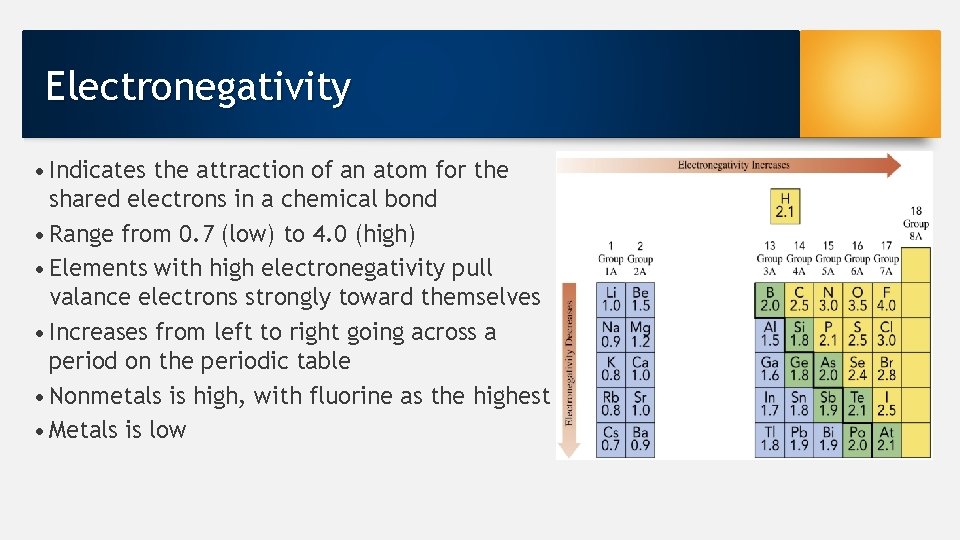
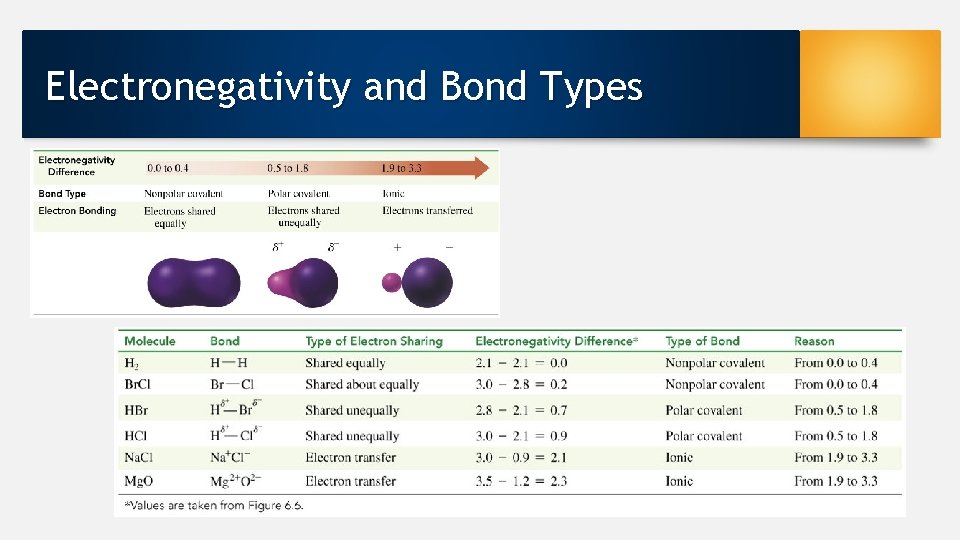
Electronegativity • Indicates the attraction of an atom for the shared electrons in a chemical bond • Range from 0. 7 (low) to 4. 0 (high) • Elements with high electronegativity pull valance electrons strongly toward themselves • Increases from left to right going across a period on the periodic table • Nonmetals is high, with fluorine as the highest • Metals is low

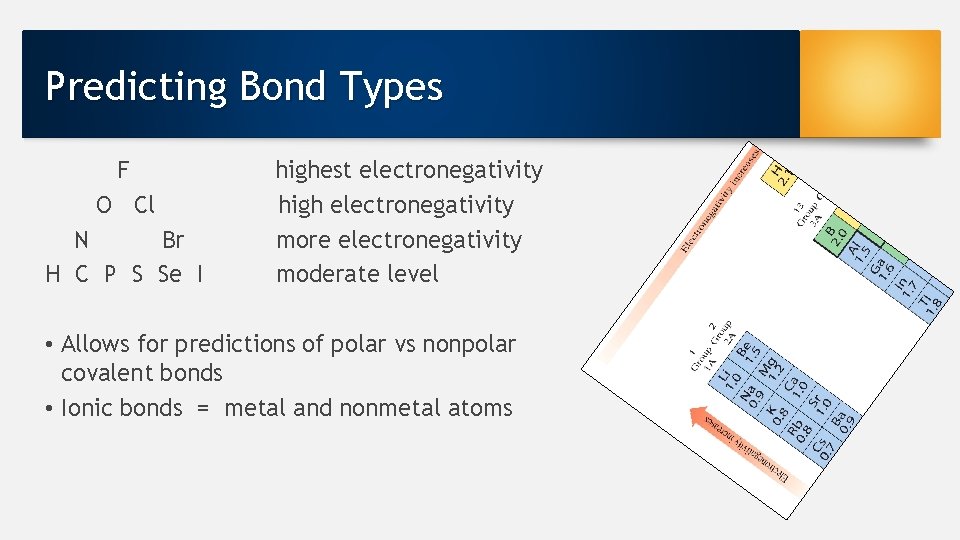
Predicting Bond Types F O Cl N Br H C P S Se I highest electronegativity high electronegativity more electronegativity moderate level • Allows for predictions of polar vs nonpolar covalent bonds • Ionic bonds = metal and nonmetal atoms

Electronegativity and Bond Types

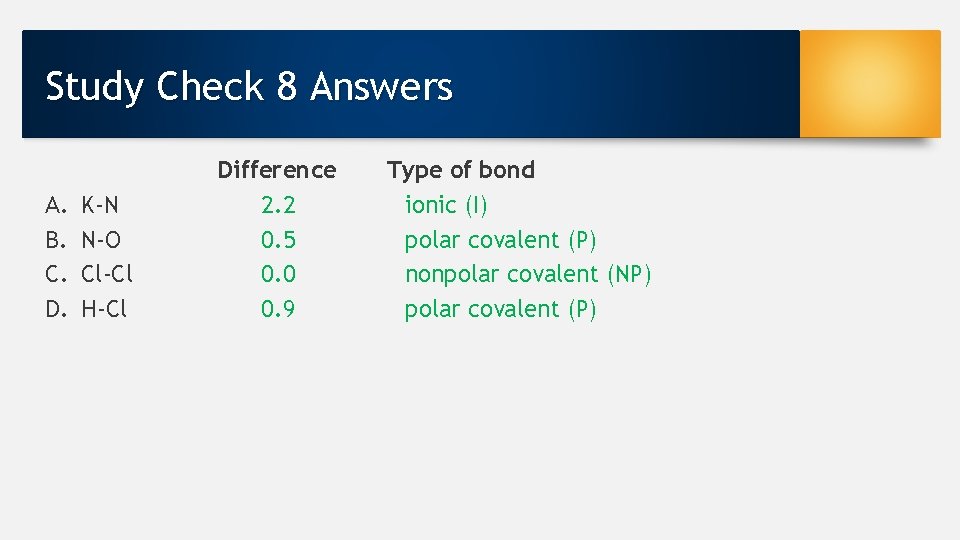
Study Check 8 Electronegativity difference to identify the type of bond [nonpolar covalent (NP), polar covalent (P), or ionic (I)] between the following: A. B. C. D. K-N N-O Cl-Cl H-Cl

Study Check 8 Answers A. B. C. D. K-N N-O Cl-Cl H-Cl Difference 2. 2 0. 5 0. 0 0. 9 Type of bond ionic (I) polar covalent (P) nonpolar covalent (NP) polar covalent (P)

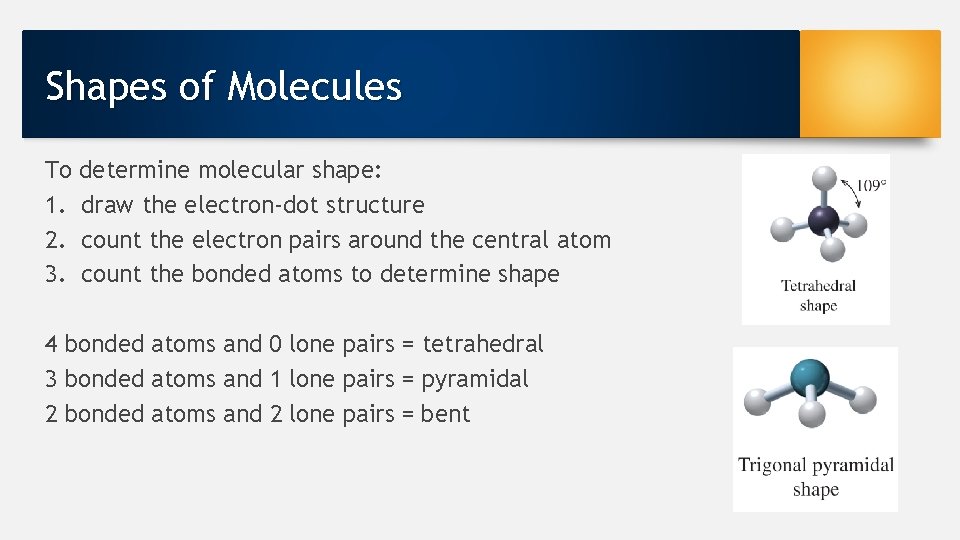
Shapes of Molecules To 1. 2. 3. determine molecular shape: draw the electron-dot structure count the electron pairs around the central atom count the bonded atoms to determine shape 4 bonded atoms and 0 lone pairs = tetrahedral 3 bonded atoms and 1 lone pairs = pyramidal 2 bonded atoms and 2 lone pairs = bent

Attractive Forces and Melting Points • Of compounds are related to the strength of attractive forces between molecules or compounds • Are lower due to weak forces such as dispersion forces • Are higher due to stronger attractive forces such as hydrogen bonding • Are highest in ionic compounds due to the strong attractive forces between ions in the compound