Ionic and Covalent Bonds Mrs Pagano Physical Science

Ionic and Covalent Bonds Mrs. Pagano Physical Science 10 CP
How Atoms Bond § Atoms bond when their valence electrons interact § Atoms join to form bonds so that they have a stable electron configuration (like the Noble Gasses)
When Atoms Bond They Form Matter § Everything in our world is the result of atoms bonding together to build compounds

There are Two Main Types of Bonds: Ionic and Covalent Bonds § Both Ionic and Covalent bonds hold atoms together to form compounds
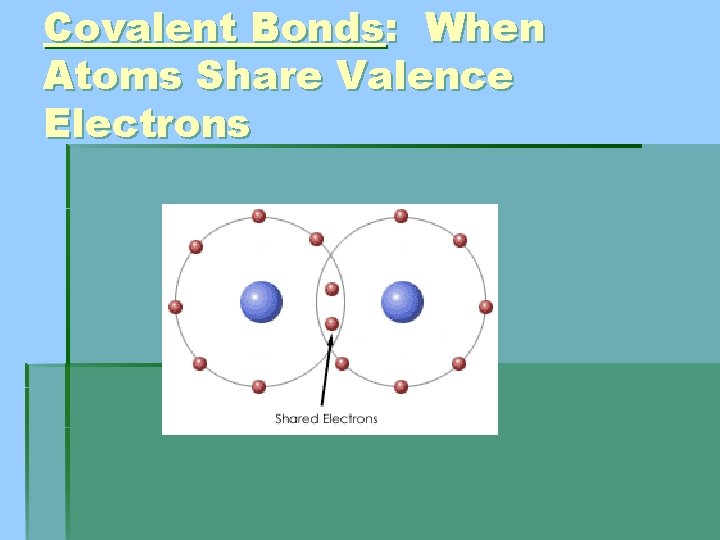
Covalent Bonds: When Atoms Share Valence Electrons
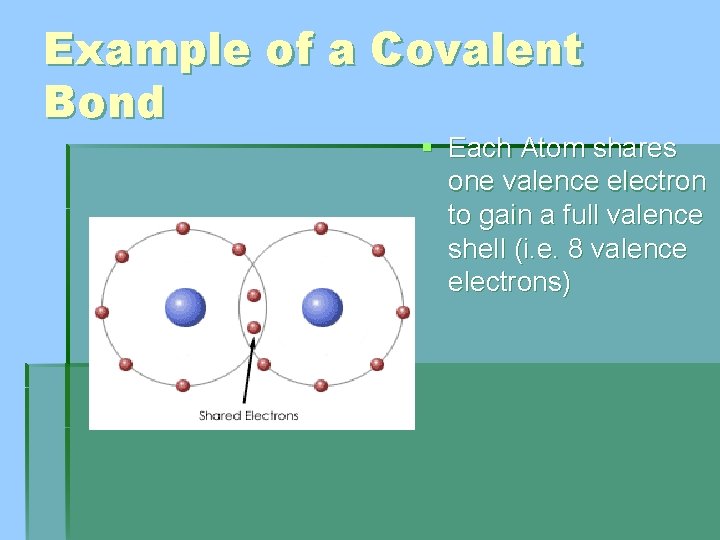
Example of a Covalent Bond § Each Atom shares one valence electron to gain a full valence shell (i. e. 8 valence electrons)
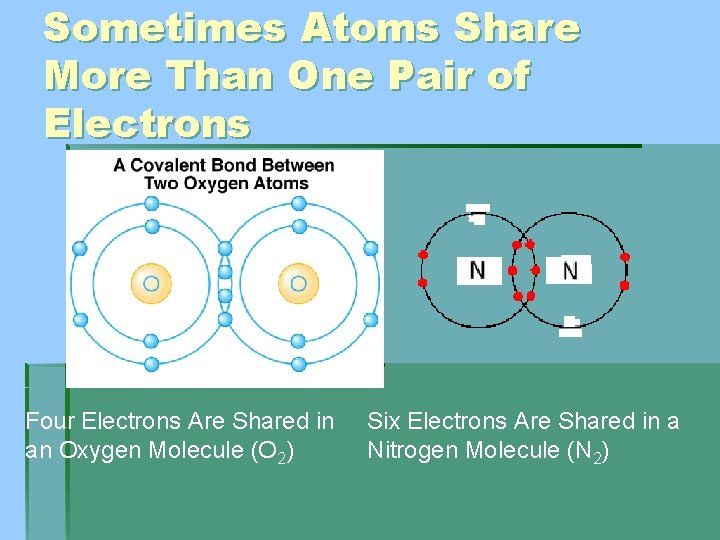
Sometimes Atoms Share More Than One Pair of Electrons Four Electrons Are Shared in an Oxygen Molecule (O 2) Six Electrons Are Shared in a Nitrogen Molecule (N 2)
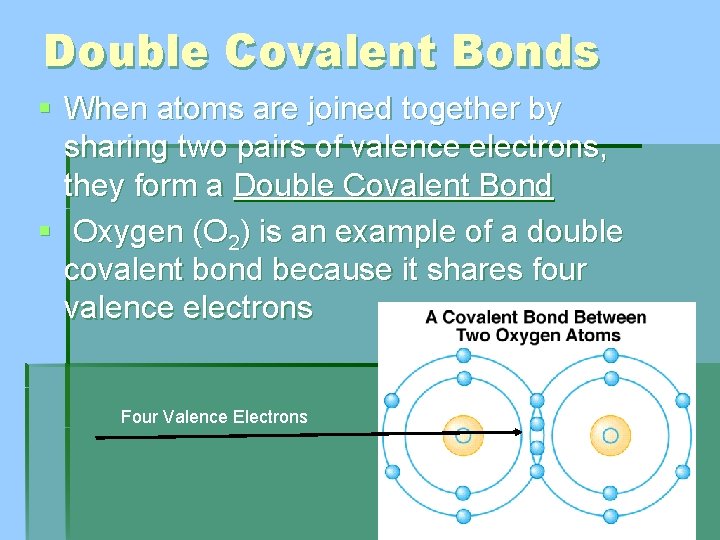
Double Covalent Bonds § When atoms are joined together by sharing two pairs of valence electrons, they form a Double Covalent Bond § Oxygen (O 2) is an example of a double covalent bond because it shares four valence electrons Four Valence Electrons
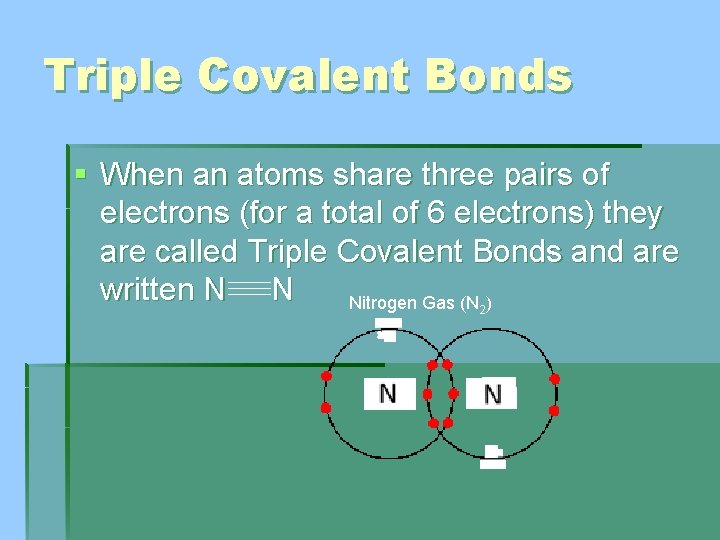
Triple Covalent Bonds § When an atoms share three pairs of electrons (for a total of 6 electrons) they are called Triple Covalent Bonds and are written N N Nitrogen Gas (N ) 2
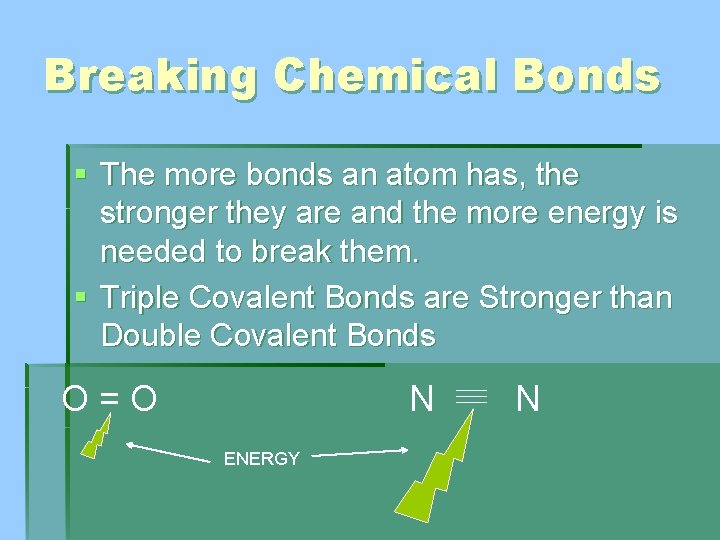
Breaking Chemical Bonds § The more bonds an atom has, the stronger they are and the more energy is needed to break them. § Triple Covalent Bonds are Stronger than Double Covalent Bonds O=O N ENERGY N
When Atoms Share Electrons Equally § When atoms share electrons equally they are called Nonpolar Covalent Bonds because each atom has a neutral charge
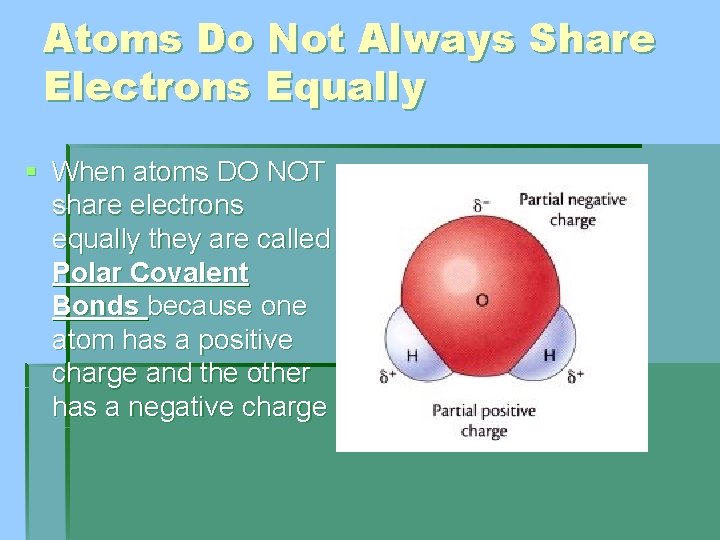
Atoms Do Not Always Share Electrons Equally § When atoms DO NOT share electrons equally they are called Polar Covalent Bonds because one atom has a positive charge and the other has a negative charge
How Do You Know If It’s a Covalent Bond? § Covalent bonds are formed by non-metal atoms § H , O , N , C , S , P , F , Cl , Br , I § If the formula of a compound contains only the symbols of nonmetals, that compound is Covalent. § For example; CH 2 O , PH 3, H 2 O, CH 3 COOH, CON 2 H 4.
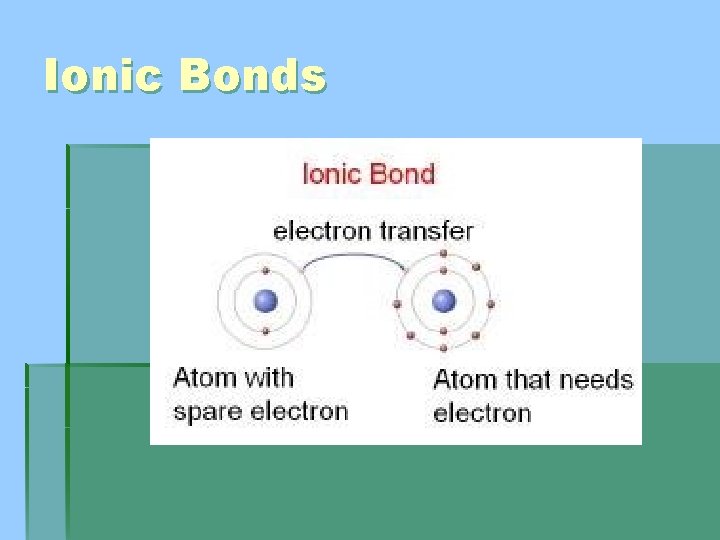
Ionic Bonds

Ionic Bonds § Ionic bonds form between ions that have opposite charges § Remember that most metals will form positively charged ions (Cations) because they will lose valence electrons and most non metals will gain electrons to form negative ions (Anions)
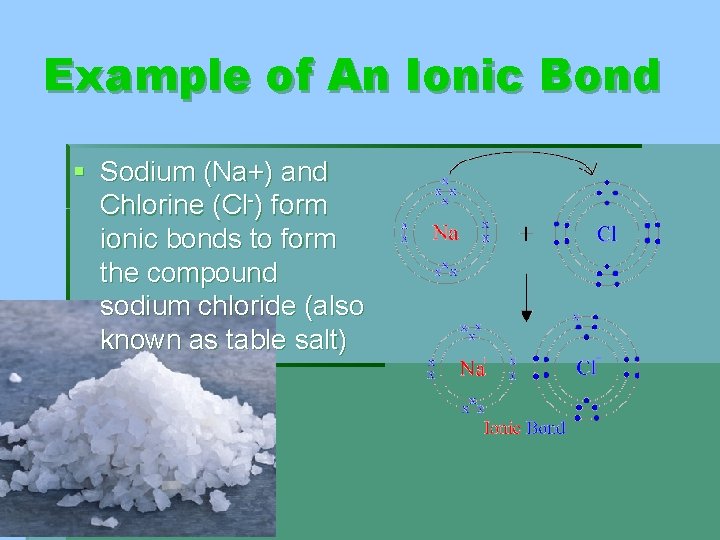
Example of An Ionic Bond § Sodium (Na+) and Chlorine (Cl-) form ionic bonds to form the compound sodium chloride (also known as table salt)
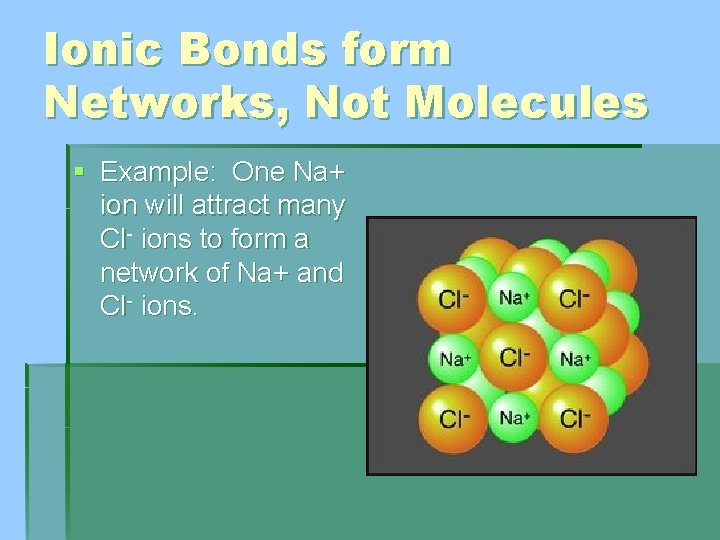
Ionic Bonds form Networks, Not Molecules § Example: One Na+ ion will attract many Cl- ions to form a network of Na+ and Cl- ions.

These networks eventually form Crystals

How Do You Know If It’s an Ionic Bond? § If in any formula , you see only ONE symbol of a metal you know it is an ionic compound. For example: Li 2 Cr 2 O 7 : both Li and Cr are metals. Cu. SO 4 : only Cu is metal. Na. NO 2 : only Na is metal. § These are all ionic compounds because they have at least one atom that is a metal in the compound
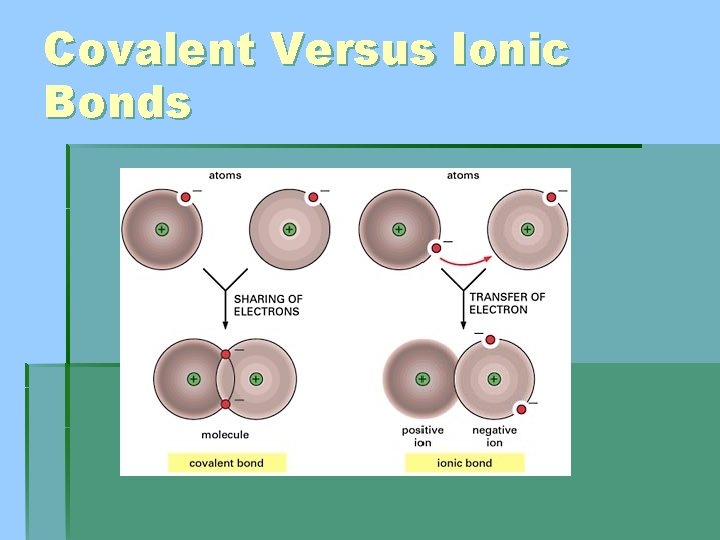
Covalent Versus Ionic Bonds
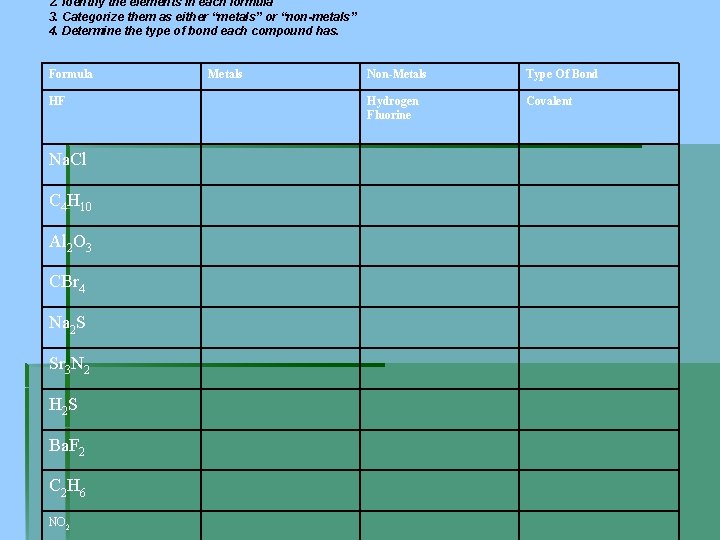
2. Identify the elements in each formula 3. Categorize them as either “metals” or “non-metals” 4. Determine the type of bond each compound has. Formula HF Na. Cl C 4 H 10 Al 2 O 3 CBr 4 Na 2 S Sr 3 N 2 H 2 S Ba. F 2 C 2 H 6 NO 2 Metals Non-Metals Type Of Bond Hydrogen Fluorine Covalent
- Slides: 21