Ionic and Covalent Bonds CHAPTER 6 Atomic Bonding

Ionic and Covalent Bonds CHAPTER 6

Atomic Bonding Why do atoms bond (react) To obtain a complete outer energy shell What does the atom do to accomplish this? Gain electrons Lose electrons Share electrons
Ionic Bonds A transfer of electrons (gaining and losing) Bond formed when opposite charged atoms (ions) are attracted each other Formed between a metal and a nonmetal Recall the valence and oxidation numbers of atoms

Example: Sodium & Chlorine Electron jumps
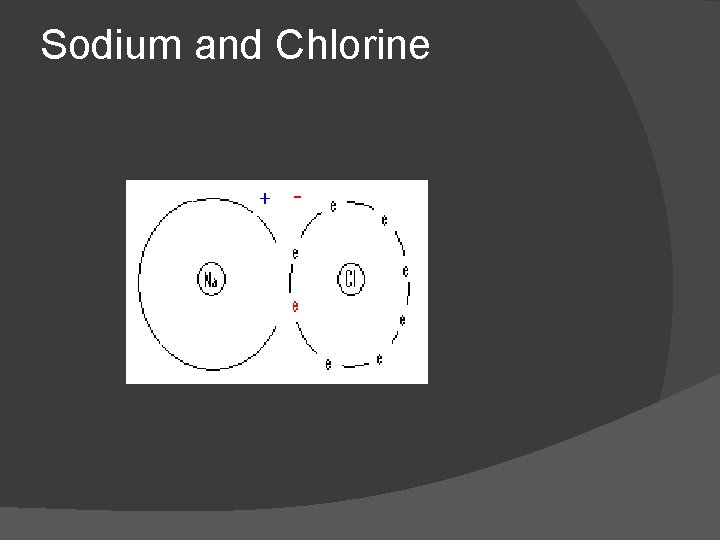
Sodium and Chlorine

Many atoms: Crystal Lattice Structure (repeating pattern)
Ionic Bonds Weak bonds (can be easily separated) Form a crystal lattice structure
Covalent Bonds Sharing of electrons Occurs between two atoms that want to gain electrons (non-metals) Very stable and strong bond

Example: Water Oxygen wants 2 e Hydrogen wants 1 e
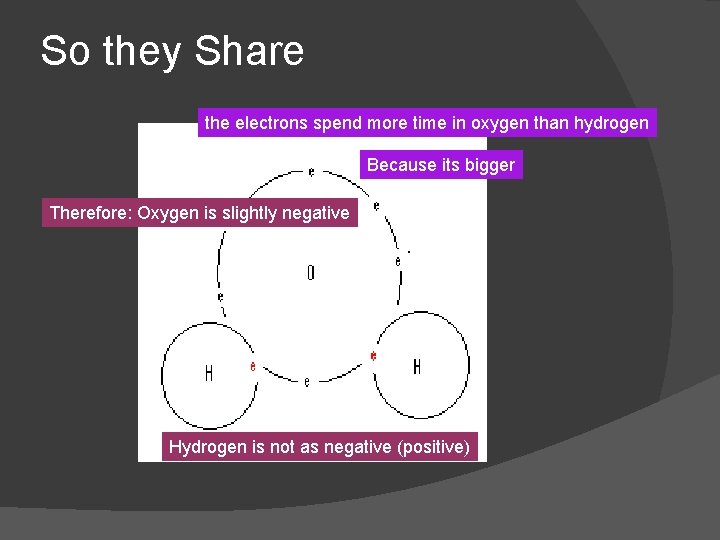
So they Share the electrons spend more time in oxygen than hydrogen Because its bigger Therefore: Oxygen is slightly negative Hydrogen is not as negative (positive)

Example Diatomic Elements A covalent bond between two atoms of the same element These elements do not like to be alone so if another element is not available they bond to themselves Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine and Iodine H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2
- Slides: 11