Ionic and Covalent Bonding Ionic Transfer Covalent Share

Ionic and Covalent Bonding Ionic Transfer Covalent Share

What holds bonded atoms together? • Bond when valence electrons interact • The (+) nucleus of one atom attracts the (-) ion from another like magnets
Bonds can bend and stretch • Bonds are not really rigid, like the stick in ball and stick models • They act more like springs.
Ionic Bonds Ionic bonds are a transfer of electrons • Ionic Bonds have positively charged ions attracted to negatively charged ion. • Cations (+) are positive, metals • CAT PAWS CATions are PAWsitive *Anions (-) are negative, non-metals • Metal + Non-Metal have ionic bonds and are therefore call Ionic Compounds
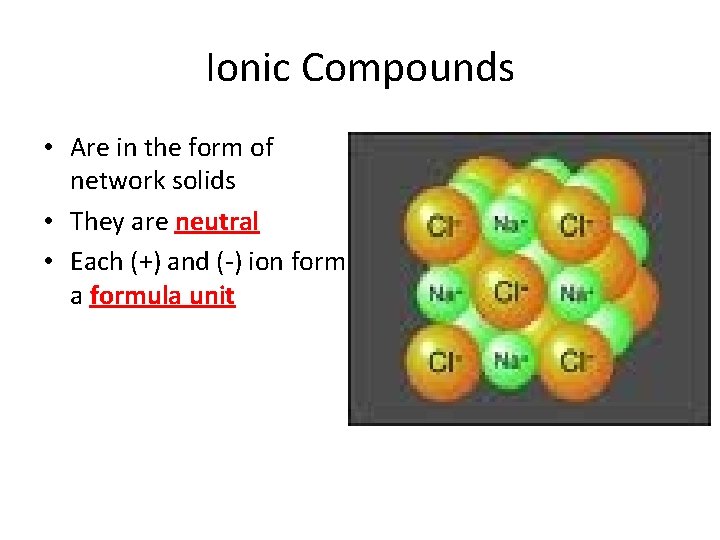
Ionic Compounds • Are in the form of network solids • They are neutral • Each (+) and (-) ion form a formula unit
Characteristics of Ionic Compounds • High Melting points • • • Strong Bonds Brittle Dissolve in water Solid states do not conduct electricity Liquid state does conduct electricity
Metallic Bonds • Conduct Electricity • Flexible • Ductile • Malleable • “Sea” of electrons formed
Covalent Bonding A covalent bond is formed by a shared pair of electrons between two atoms.

Molecules Share more than 1 pair of electrons • Bonds may be…. . • Single 1 shared electron pair represented by 1 line. Requires some energy to break • Double 2 shared electron pair represented by 2 lines. Requires more energy to break • Triple-3 Triple shared electron pair represented by 3 lines. Requires the most energy to break
Polarity • Polar means two opposite ends • Not all electron pairs are shared the same • They are attracted to one nucleus more than the other • This is a polar covalent bond

Ionic Compounds • Polyatomic ions – Poly- means many or more than one – These are two or more atoms bonded together with an overall charge – Most polyatomic ions are negative – Parentheses will group polyatomics in a compound Mg(NO 3)2 – An –ate ending means there is an oxygen atom involved in the polyatomic ion
- Slides: 11