Ionic and Covalent bonding Chapters 15 and 16

Ionic and Covalent bonding Chapters 15 and 16

Lewis Dot Diagrams Ex) Draw the Lewis Dot diagram for oxygen (atomic #8) step # 1) determine the number of valence electrons (electrons in outer level) Oxygen is in group 6 A oxygen has 6 valence electrons Step # 2) Draw dot structure . . . O: .
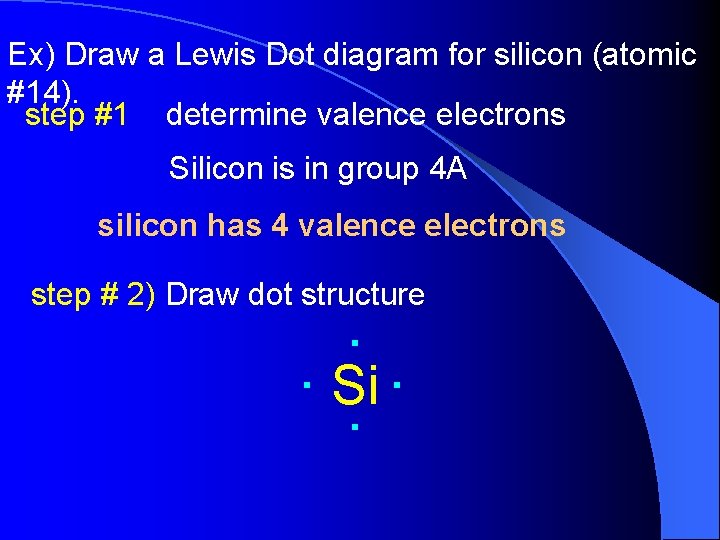
Ex) Draw a Lewis Dot diagram for silicon (atomic #14). step #1 determine valence electrons Silicon is in group 4 A silicon has 4 valence electrons step # 2) Draw dot structure . . . Si.

Octet Rule: atoms having 8 electrons in their outer level have the highest degree of stability. - electrons can be transferred (ionic bond) or shared (covalent bond) for the atom to achieve a total of eight electrons - the Noble Gases already have eight electrons which is why they are unreactive.
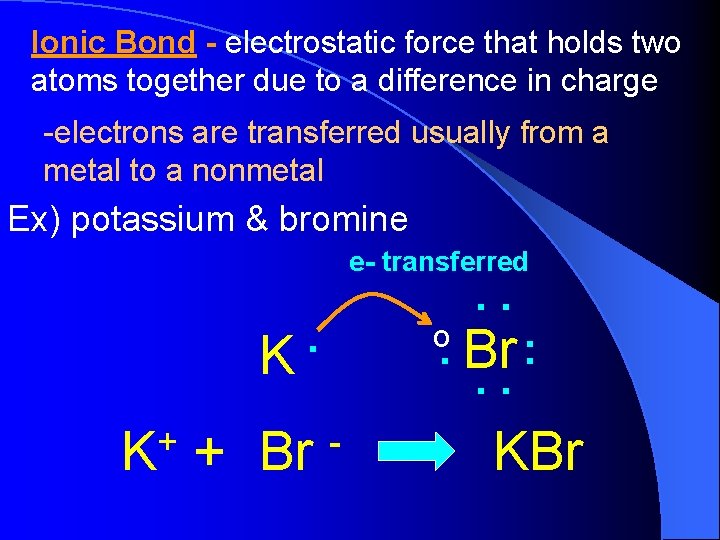
Ionic Bond - electrostatic force that holds two atoms together due to a difference in charge -electrons are transferred usually from a metal to a nonmetal Ex) potassium & bromine e- transferred . K + Br o . . . Br : . . - KBr
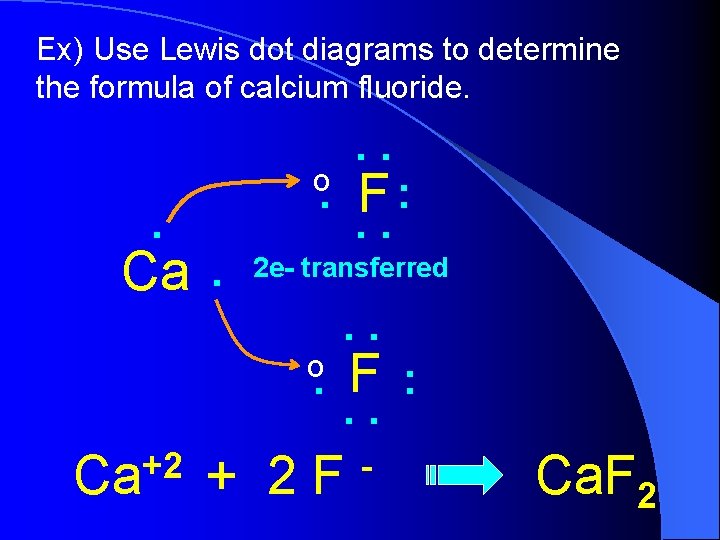
Ex) Use Lewis dot diagrams to determine the formula of calcium fluoride. . . o . Ca. 2 e- transferred o . +2 Ca : F. . . F: . . + 2 F - Ca. F 2

Properties of Ionic Compounds - are solid, generally forming crystals - have high melting points (because their bonds are so strong) - conduct electricity in the molten state and when dissolved in water Metallic Bond - chemical bond that occurs between pure metals - electrons are free to drift from atom to atom - positive charge on nucleus pulls any electron toward it, even if it is from a different atom
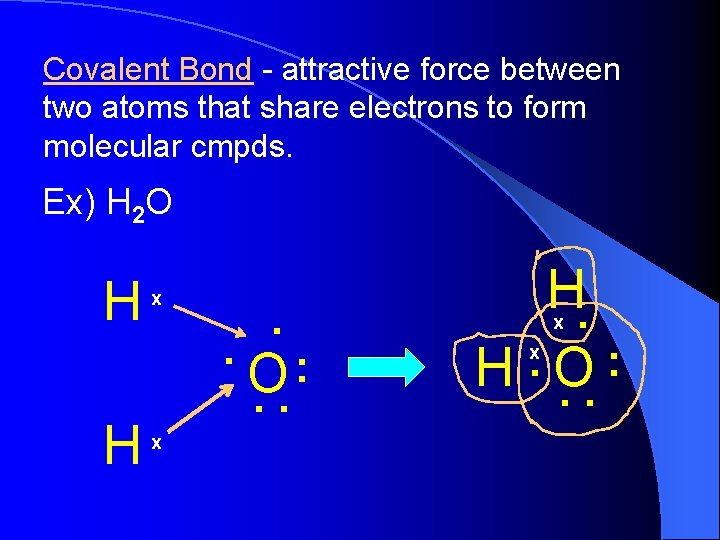
Covalent Bond - attractive force between two atoms that share electrons to form molecular cmpds. Ex) H 2 O H H x . : O. . H. : H. O. . x x
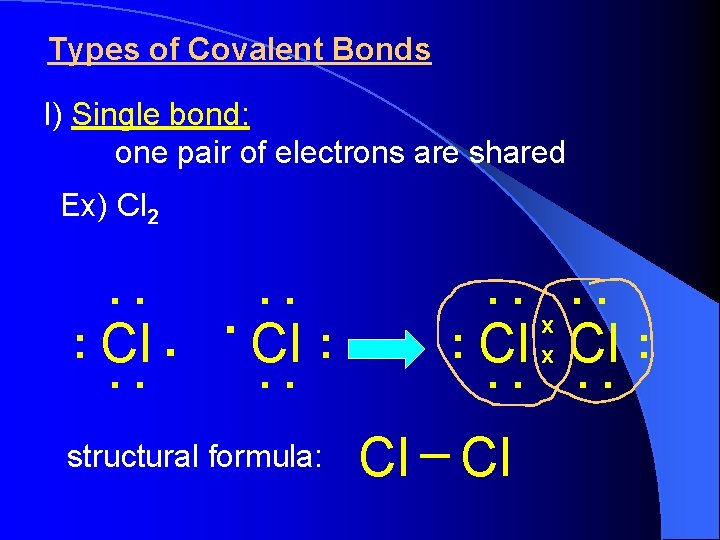
Types of Covalent Bonds I) Single bond: one pair of electrons are shared Ex) Cl 2 . . . : Cl. . structural formula: . . : Cl. . Cl Cl x x . . : Cl. .
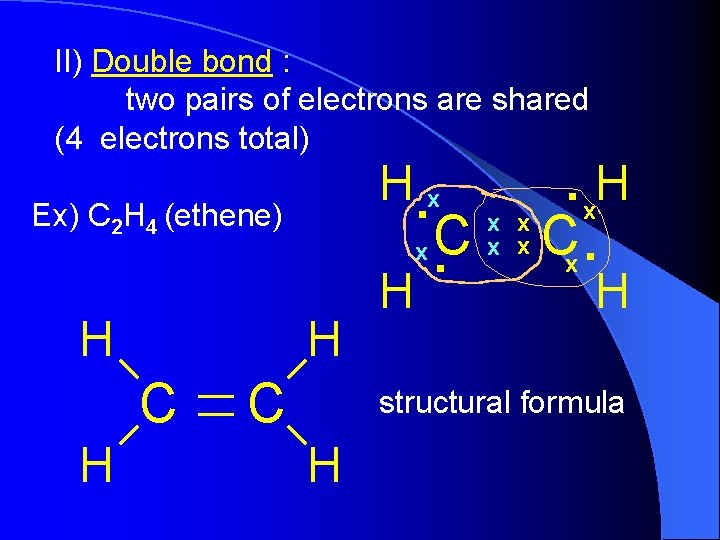
II) Double bond : two pairs of electrons are shared (4 electrons total) Ex) C 2 H 4 (ethene) H H C H . x. H x x x. C x x C x. . H. x C H H structural formula H
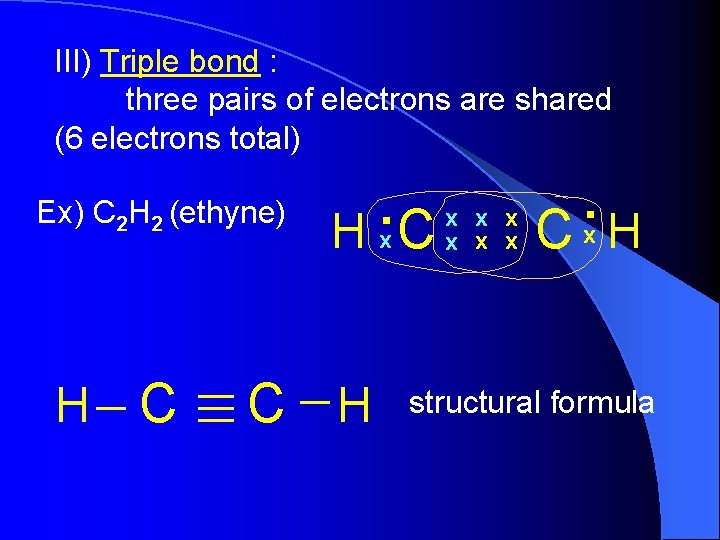
III) Triple bond : three pairs of electrons are shared (6 electrons total) Ex) C 2 H 2 (ethyne) H C . x H x. C x C H x x . x C H structural formula
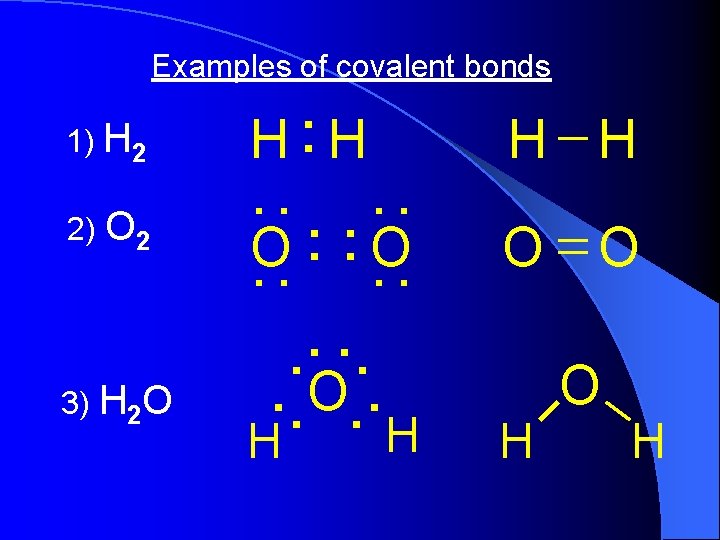
Examples of covalent bonds 1) H 2 2) O 2 3) H 2 O . . H H. . . . O. . H H O O H
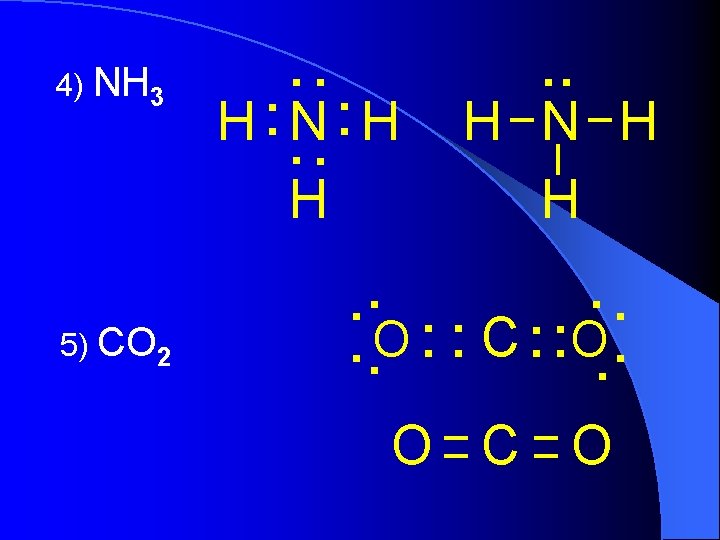
4) NH 3 5) CO 2 . . . H N H H . . O. . C. . O C O
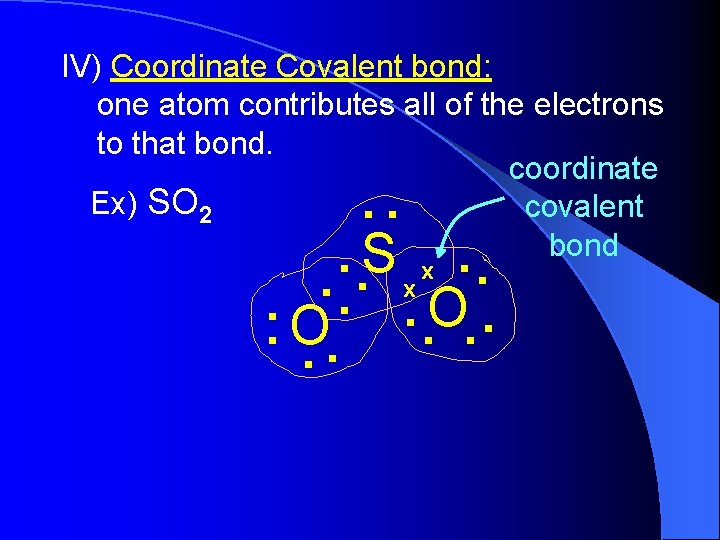
IV) Coordinate Covalent bond: one atom contributes all of the electrons to that bond. coordinate Ex) SO 2 covalent bond . . . S. x. . O. . . .
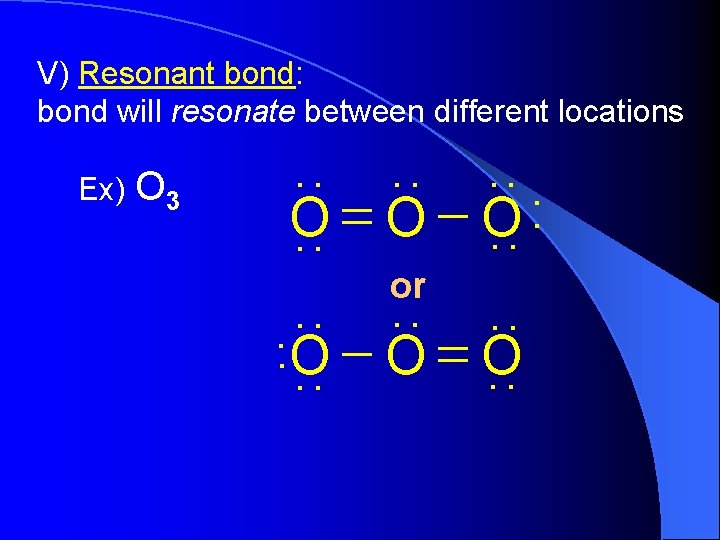
V) Resonant bond: bond will resonate between different locations. . Ex) O 3 O. . O or. . O. . . .
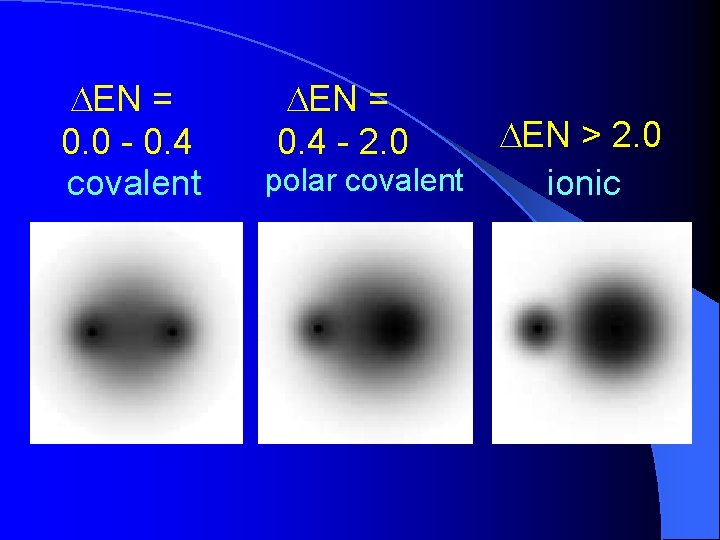
DEN = 0. 0 - 0. 4 covalent DEN = 0. 4 - 2. 0 DEN > 2. 0 polar covalent ionic
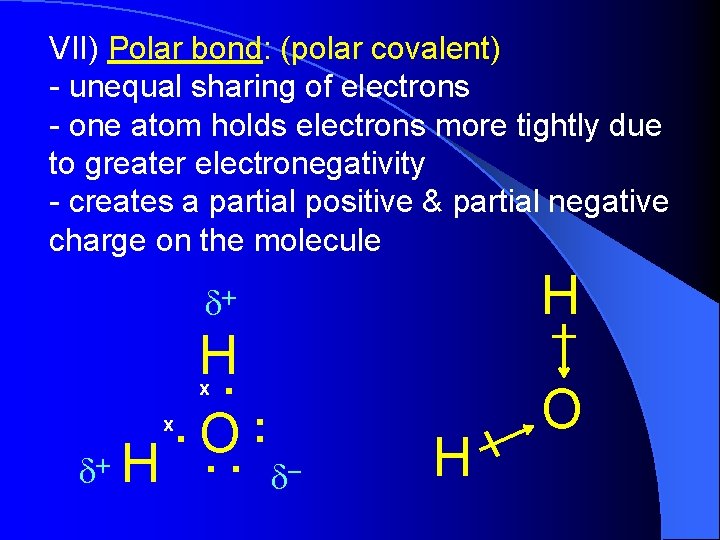
VII) Polar bond: (polar covalent) - unequal sharing of electrons - one atom holds electrons more tightly due to greater electronegativity - creates a partial positive & partial negative charge on the molecule H d+ H. . O: . . x d+ H x d- H O
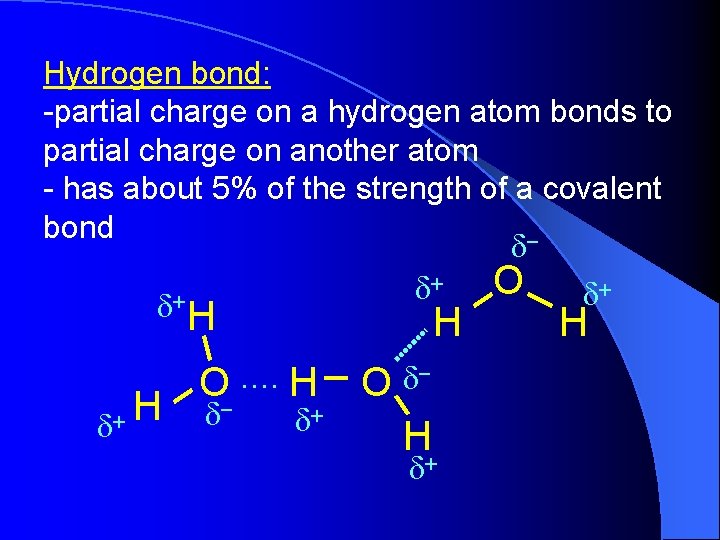
Hydrogen bond: -partial charge on a hydrogen atom bonds to partial charge on another atom - has about 5% of the strength of a covalent bond d+ O d+ d H d+ H H O. . H d- d+ O d- H+ d H
- Slides: 18