Ion Channels Lecture 1 Membrane Potentials Ion Movement

• Ion Channels Lecture 1 –Membrane Potentials: Ion Movement - Forces and Measurement

The relative permeability of a lipid bilayer to different classes of molecules Alberts et al.
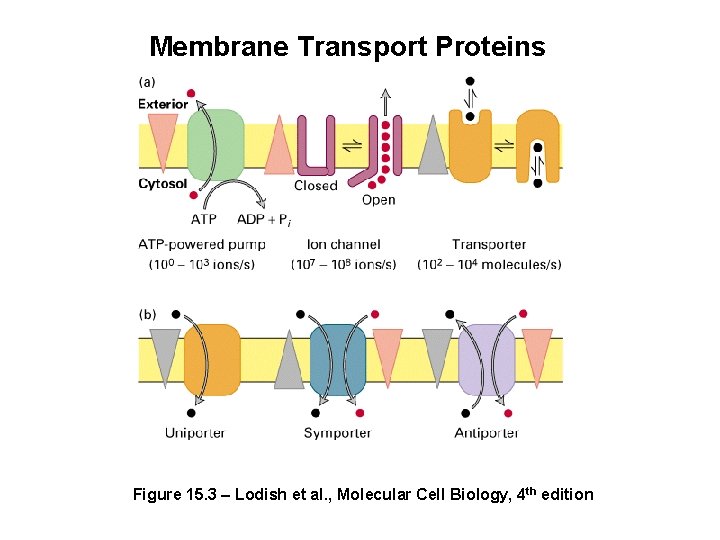
Membrane Transport Proteins Figure 15. 3 – Lodish et al. , Molecular Cell Biology, 4 th edition
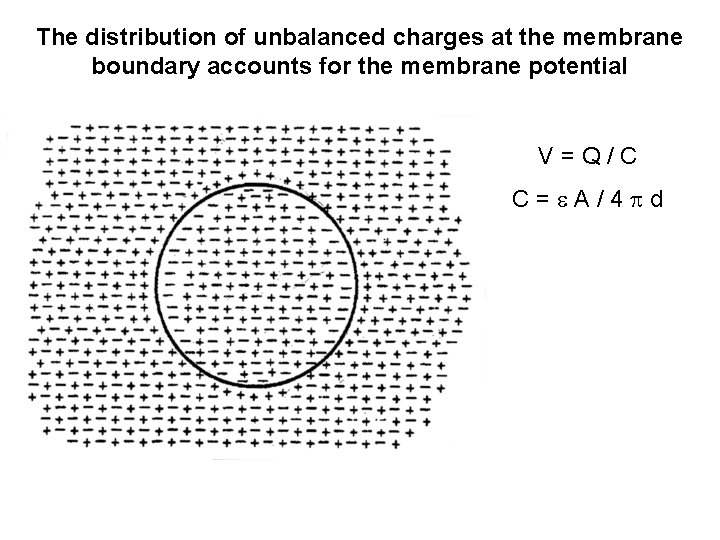
The distribution of unbalanced charges at the membrane boundary accounts for the membrane potential V=Q/C C= A/4 d

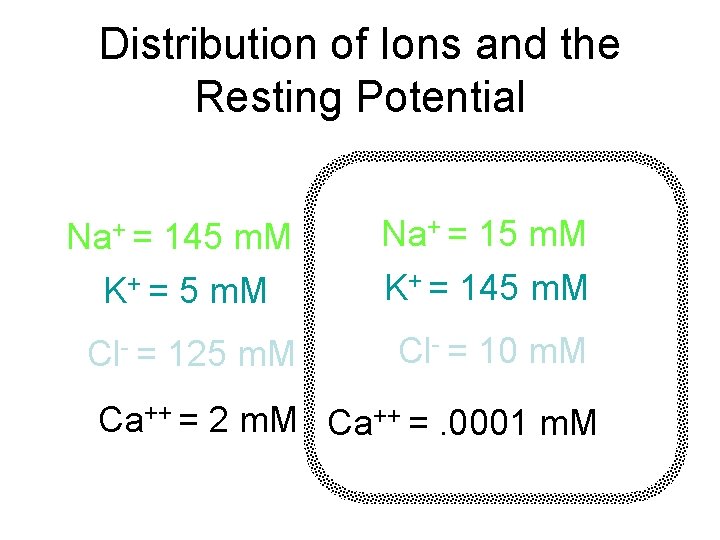
Distribution of Ions and the Resting Potential Na+ = 15 m. M K+ = 145 m. M Cl- = 10 m. M 145 m. M K+ = 5 m. M 125 m. M Ca++ = 2 m. M Ca++ =. 0001 m. M

Understanding the Nernst potential 1. EK is the Nernst potential for K ions. It is often called the equilibrium potential for K. 2. Equilibrium means there is no net movement of K ions across the membrane. This occurs when there is no energy difference between inside and outside. 3. Two kinds of energy are important: chemical and electrical Chemical energy = o+RTln. C per mole of ion Electrical energy = z. FV per mole of ion where F is the Faraday constant 96, 500 coulombs/mole or 23, 602 cal mol-1 V-1

Understanding the Nernst potential (2) 4. At equilibrium, the total energy (chemical + electrical) is the same on both sides of the membrane. In other words, the chemical energy difference across the membrane is counterbalanced by an equal and opposite electrical energy difference. 5. Consider a membrane, set up with concentrated KCl on the left and dilute KCl on the right. Now let the membrane suddenly become permeable to K ions. A tiny amount of K leaves the concentrated side and enters the dilute side. This leads to charge separation, and a voltage difference across the membrane.
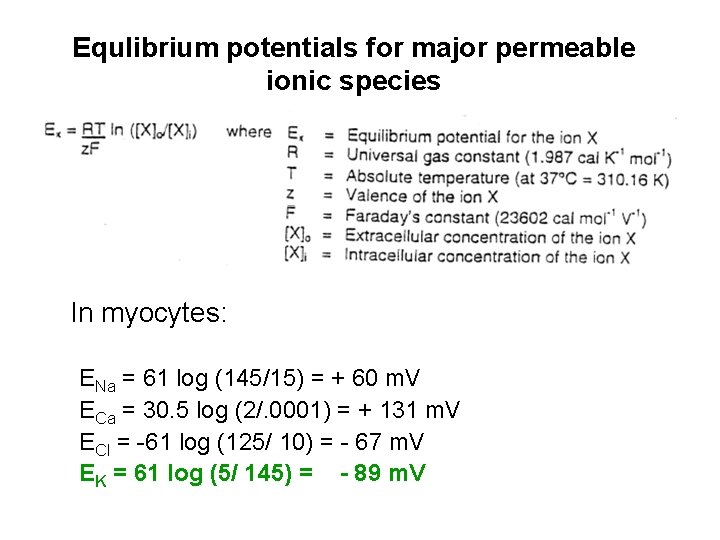
Equlibrium potentials for major permeable ionic species In myocytes: ENa = 61 log (145/15) = + 60 m. V ECa = 30. 5 log (2/. 0001) = + 131 m. V ECl = -61 log (125/ 10) = - 67 m. V EK = 61 log (5/ 145) = - 89 m. V
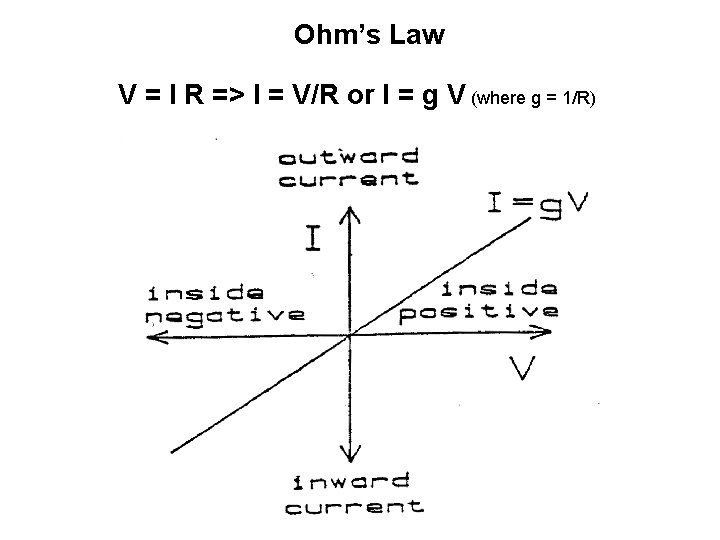
Ohm’s Law V = I R => I = V/R or I = g V (where g = 1/R)

The direction of ion movement An example: • Chemical vs electrical force and Net force • Ohms law • Rule of thumb: Whenever Vm is more negative than Ex, the current is inward. Whenever Vm is more positive than Ex, the current is outward. At Ex the current is zero (i. e. equilibrium) • Whenever an ion channel opens and ions move down the electrochemical gradient, the membrane potential will move towards the equilibrium potential for that ion
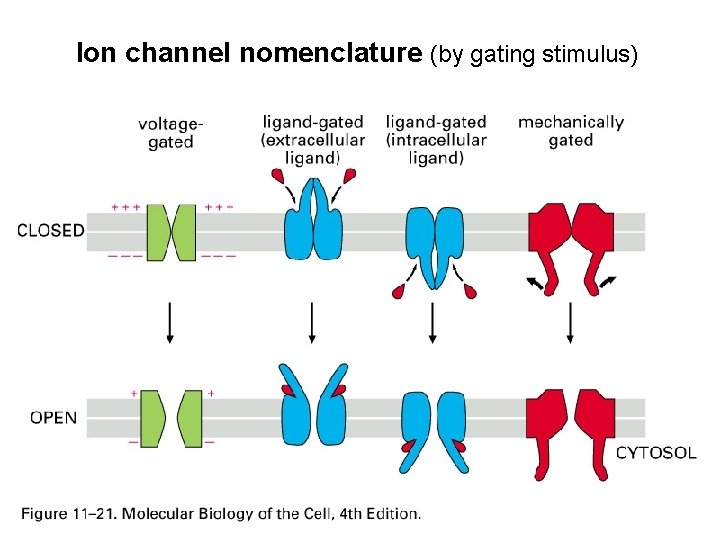
Ion channel nomenclature (by gating stimulus)
- Slides: 12