InVivo Therapeutics Scaffold for Acute SCI Presented by
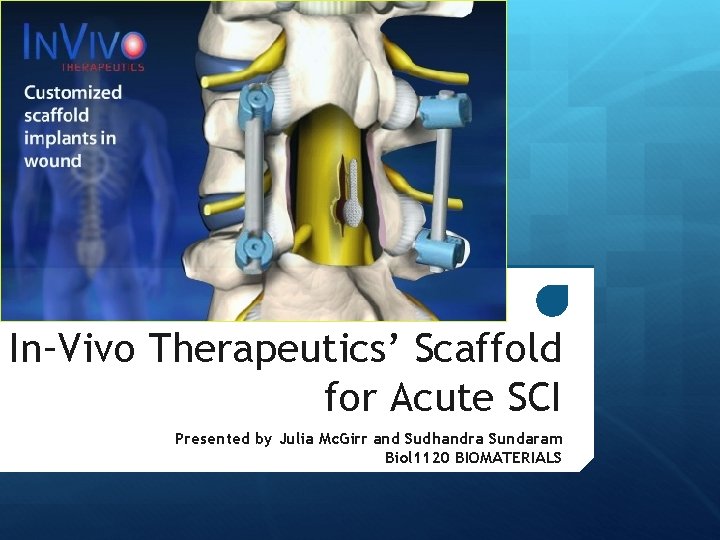
In–Vivo Therapeutics’ Scaffold for Acute SCI Presented by Julia Mc. Girr and Sudhandra Sundaram Biol 1120 BIOMATERIALS

Overview of Spinal Cord Injury (SCI) Injury to spinal cord is often caused by trauma Most commonly: motor vehicle accident, falls, sports injuries, lateral bending, dislocation, rotation, axial loading, and other types of major trauma Depending on the location of the injury, symptoms can vary from pain to paralysis The type of injury is determined by the area of the body that the injured area of the spine innervates For example, if a section of skin is innervated by a specific part of the spine (dermatome), a SCI can cause pain, numbness, or a loss of sensation in the corresponding areas Another example, group of innervated muscles (myotome) injury can cause voluntary motor control problems
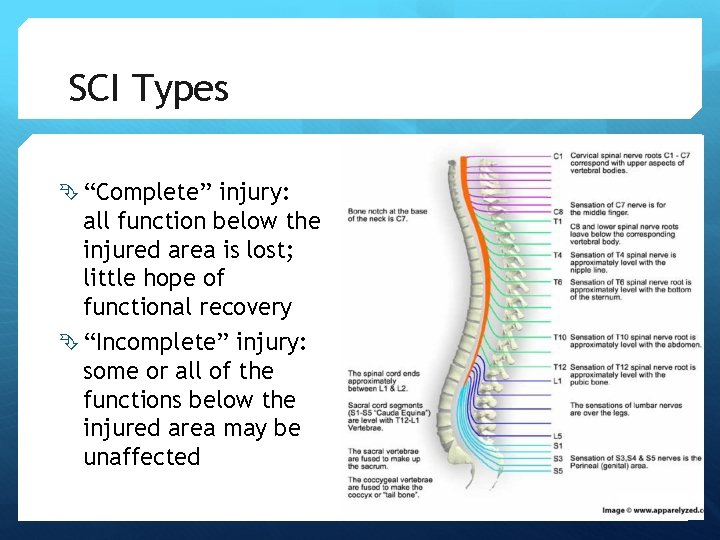
SCI Types “Complete” injury: all function below the injured area is lost; little hope of functional recovery “Incomplete” injury: some or all of the functions below the injured area may be unaffected
Complications and Diagnosis of SCI Neurogenic shock, respiratory failure, pulmonary edema, pneumonia, pulmonary emboli, deep venous thrombosis Diagnosis X-ray, MRI, CT scan to determine if there is any damage to the spinal cord and WHERE it is located Neurologic evaluation
Current Treatment Modern trauma care includes clearing the cervical spine – person with a suspected injury is treated as if they have an injury Immobilized at the scene of the injury until it is clear that there is no damage to the highest spine portions (long spine board/hard collar) Patients REQUIRE extended treatment in the ICU One experimental treatment: therapeutic hypothermia is used but no evidence that it improves outcome Another treatment with little evidence of effectiveness: maintenance of adequate blood supply to nerves
SCI Surgery and Drugs Surgery may be necessary to remove any bone fragments from the spinal canal and to stabilize the spine Anti-inflammatory drug treatment (i. e. methylprednisolone) High dose of methyprednisolone may improve outcome Studies are not vey promising and there is an increased risk of serious infection or sepsis due to immunosuppressive qualities of high-dose corticosteroids

Rehabilitation One of the few treatment possibilities for acute and chronic SCI Acute care setting is the first step Team of healthcare workers Respiratory status, prevention of indirect complications, maintaining range of motion, keeping musculature active Great emphasis on airway clearance
Prognosis SCI results in at least some incurable impairment even with the best possible treatment Recovery is quickest during the first 6 months After this point, outcome is not promising These implantable scaffolds by In-Vivo Therapeutics are a definite breakthrough
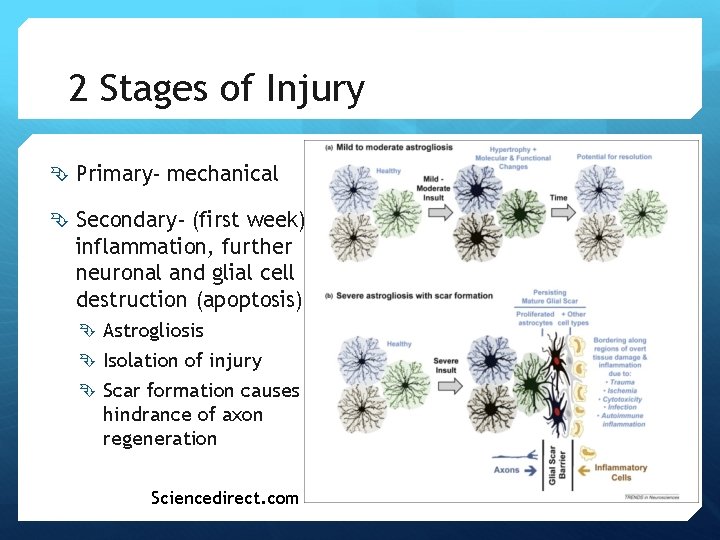
2 Stages of Injury Primary- mechanical Secondary- (first week) inflammation, further neuronal and glial cell destruction (apoptosis) Astrogliosis Isolation of injury Scar formation causes hindrance of axon regeneration Sciencedirect. com
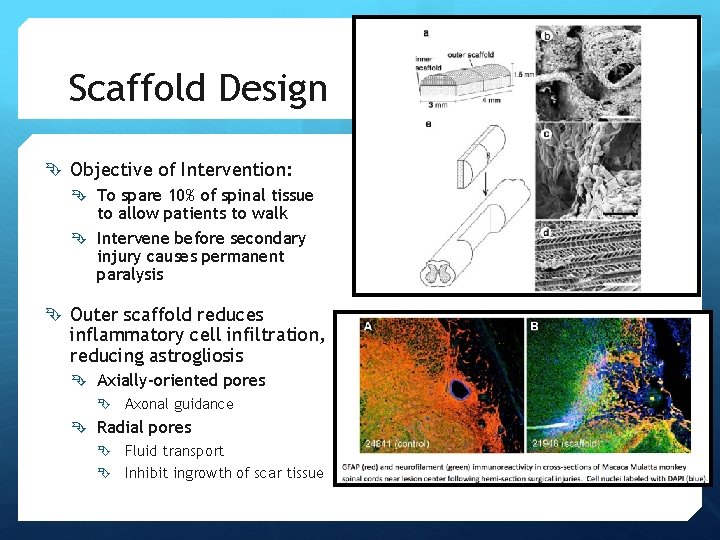
Scaffold Design Objective of Intervention: To spare 10% of spinal tissue to allow patients to walk Intervene before secondary injury causes permanent paralysis Outer scaffold reduces inflammatory cell infiltration, reducing astrogliosis Axially-oriented pores Axonal guidance Radial pores Fluid transport Inhibit ingrowth of scar tissue
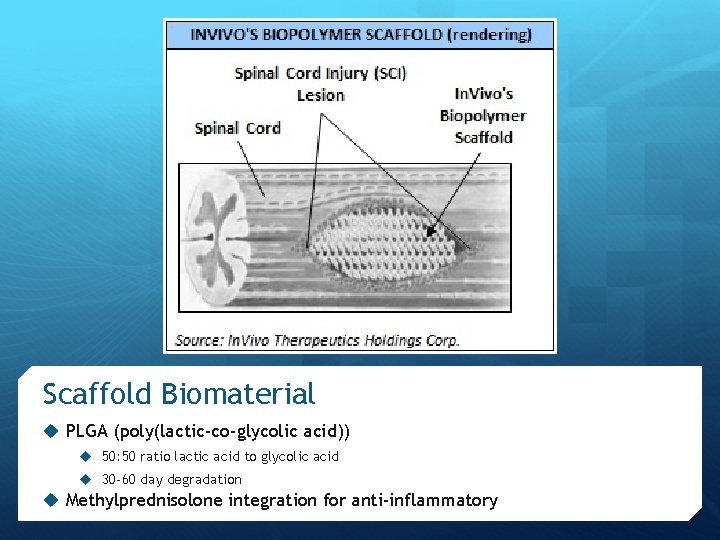
Scaffold Biomaterial u PLGA (poly(lactic-co-glycolic acid)) u 50: 50 ratio lactic acid to glycolic acid u 30 -60 day degradation u Methylprednisolone integration for anti-inflammatory
Practical Information Forecasted Cost: $60, 000/unit but could exceed $100, 000/unit Should reduce therapy cost by 80% Therapeutic Procedures 1 min 4 sec for customization and implantation For Scaffold: Pedical screws implanted, scaffold sized and inserted through surgery For Hydrogel: Radiographic-guided syringe, non-invasive For Injectable Scaffold: Tuohy needle Recovery Procedures Rest, avoid application of pressure

Scaffolding Device: Insurance Coverage This type of therapy is likely not covered by insurance because it is investigational Some private health insurance companies may help with the costs Information is scarce about this, but coverage depends on the type of insurance a patient has About 52% of patients are covered by private health insurance at the time of injury, helping with payments This device is the first implant for spinal cord injury, thus making this a unique case Exact insurance policies on the device are unknown
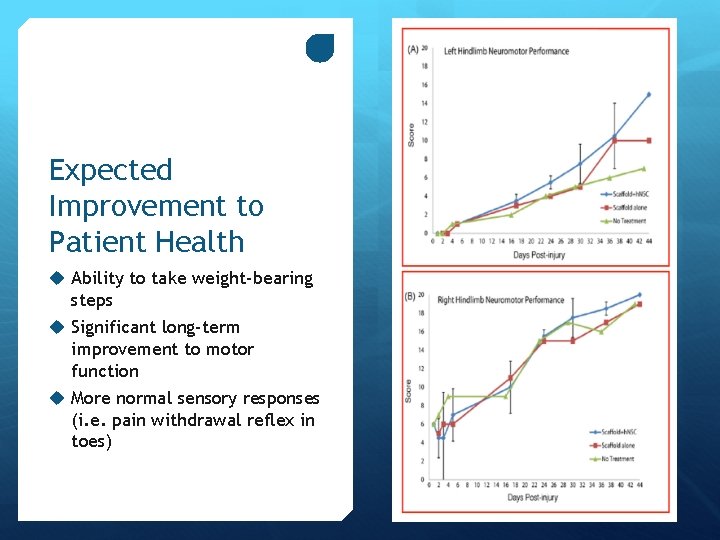
Expected Improvement to Patient Health u Ability to take weight-bearing steps u Significant long-term improvement to motor function u More normal sensory responses (i. e. pain withdrawal reflex in toes)
Expected Complications Risk of Infection at Surgical Site Hypersensitivity Possibility of dislodgement/misplace ment Pro: Autologous Stem Cells (no rejection risk)
References Taber, Clarence Wilbur; Venes, Donald (2009). Taber's cyclopedic medical dictionary. F. A. Davis. pp. 2173– 4. Lin VWH; Cardenas DD; Cutter NC; Frost FS; Hammond MC (2002). Spinal Cord Medicine: Principles and Practice. Demos Medical Publishing. Ho C. H. , Wuermser L. A. , Priebe M. M. , Chiodo A. E. , Scelza W. M. , Kirshblum S. C. (2007). Spinal cord injury medicine. 1. Epidemiology and classification. Archives of Physical Medicine and Rehabilitation 88: S 49 -S 54. Andrew B. , MD Peitzman; Andrew B. Peitzman; Michael, MD Sabom; Donald M. , MD Yearly; Timothy C. , MD Fabian (2002). The trauma manual. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 140– 56. Bracken MB (2012). Steroids for acute spinal cord injury. Cochrane Database Syst Rev 1: CD 001046. Fulk G; Schmitz T; Behrman A (2007). "Traumatic Spinal Cord Injury". In O'Sullivan S; Schmitz T. Physical Rehabilitation (5 th ed. ). Philidelphia Pennsylvania: F. A. Davis. pp. 937– 96.

References (cont. ) Zhang et al. "Inflammation & apoptosis in spinal cord injury. " Indian Journal of Medical Research. 135. 3 (2012): 287– 296. Print. <http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3361863/>. Teng et al. "Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. " PNAS. 99. 5 (2002): n. page. Print. <http: //invivotherapeutics. com/pdf/Teng_Langer_Polymer_Stem. Cell_2002. pdf>. http: //www. invivotherapeutics. com
- Slides: 18