Investigation of Glass Materials for Pulsed Power Capacitors




- Slides: 21

Investigation of Glass Materials for Pulsed Power Capacitors Sponsored by John Luginsland, AFOSR/RTB 7 Jan 2013 Integrity Service Excellence PI- Ed Stutz, RXAN Co-PI Jonathan Goldstein, RXAP Materials and Manufacturing Directorate Air Force Research Laboratory Proper DISTRIBUTION STATEMENT Here contractors Distribution A: Place approved authorized for public to DOD release, and US distribution DOD unlimited. 1

Overview • Background • Bulk Synthesis of Glass for Pulsed Laser Deposition targets • Glass characterization Proper DISTRIBUTION STATEMENT Here contractors Distribution A: Place approved for public release, distribution unlimited. Distribution authorized to DOD and US DOD 2

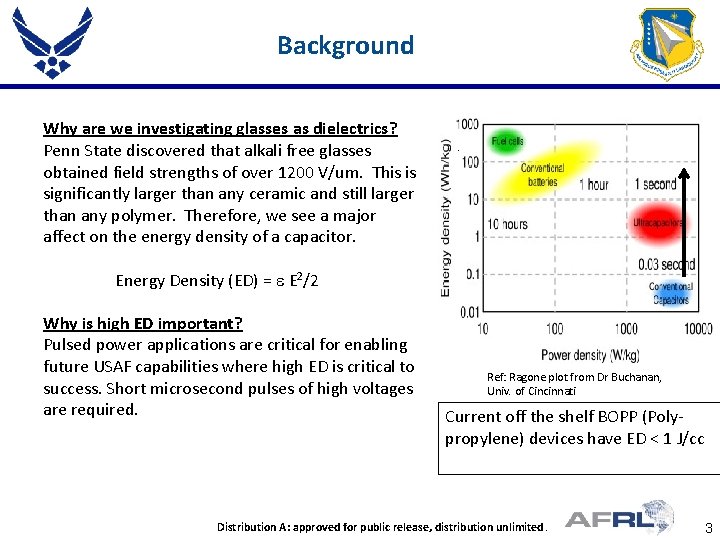
Background Why are we investigating glasses as dielectrics? Penn State discovered that alkali free glasses obtained field strengths of over 1200 V/um. This is significantly larger than any ceramic and still larger than any polymer. Therefore, we see a major affect on the energy density of a capacitor. Energy Density (ED) = e E 2/2 Why is high ED important? Pulsed power applications are critical for enabling future USAF capabilities where high ED is critical to success. Short microsecond pulses of high voltages are required. Ref: Ragone plot from Dr Buchanan, Univ. of Cincinnati Current off the shelf BOPP (Polypropylene) devices have ED < 1 J/cc Place Proper DISTRIBUTION STATEMENT Here Distribution A: approved fortopublic release, distribution unlimited. Distribution authorized DOD and US DOD contractors 3

Questions to be answered Glass-ceramic formation: • How do the mechanical and dielectric field strengths of the glass-ceramics and parent glasses compare to each other? Polarizable content: • By what factor does higher polarizable content increase the energy storage capacity? • Does higher polarizable content have negative implications for the dielectric field strength? Thickness and surface quality: • How does the morphology and dielectric field strength of a PLD-deposited film compare with that of a bulk glass? • Do post-deposition processes such as flame-polishing improve the surface quality and/or dielectric breakdown strength of PLD films? Self healing Can we improve the self-healing characteristic of glasses? Place Proper DISTRIBUTION STATEMENT Here contractors Distribution A: approved for public release, unlimited. Distribution authorized to DOD and USdistribution DOD 4

Glass Background • Glass has been around for thousands of years • Today glass has various technological uses: • Originally used in capacitor in 1744 by Ewald Georg van Kleist • Sealants in solid oxide fuel cells • Displays • AVX is the only known manufacturer of glass capacitors • Foreign owned • Capability limited to 2 n. F • Very reliable, used in many NASA missions • Relatively expensive compared to ceramics and polymers • ONR had a glass development program, but is finished now. • We are developing glass from scratch specifically designed for high power pulsed capacitor applications and advance the basic scientific understanding of issues relevant to AF glasses for capacitors Place Proper DISTRIBUTION STATEMENT Here contractors Distribution A: approved for to public release, unlimited. Distribution authorized DOD and US distribution DOD 5

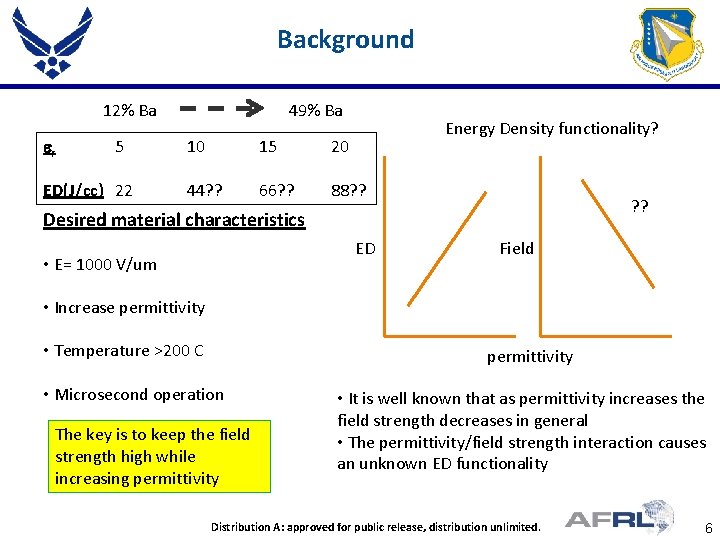
Background 12% Ba er 5 ED(J/cc) 22 49% Ba Energy Density functionality? 10 15 20 44? ? 66? ? 88? ? Desired material characteristics ED • E= 1000 V/um Field • Increase permittivity • Temperature >200 C permittivity • Microsecond operation The key is to keep the field strength high while increasing permittivity • It is well known that as permittivity increases the field strength decreases in general • The permittivity/field strength interaction causes an unknown ED functionality Place Proper DISTRIBUTION STATEMENT Here contractors Distribution A: approved for to public release, unlimited. Distribution authorized DOD and US distribution DOD 6

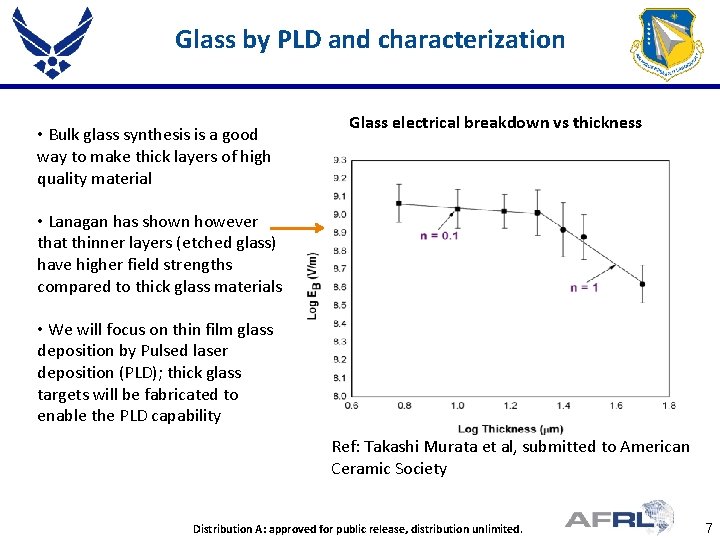
Glass by PLD and characterization • Bulk glass synthesis is a good way to make thick layers of high quality material Glass electrical breakdown vs thickness • Lanagan has shown however that thinner layers (etched glass) have higher field strengths compared to thick glass materials • We will focus on thin film glass deposition by Pulsed laser deposition (PLD); thick glass targets will be fabricated to enable the PLD capability Ref: Takashi Murata et al, submitted to American Ceramic Society Place Proper DISTRIBUTION STATEMENT Here contractors Distribution A: approved authorized for public to DOD release, anddistribution US DOD unlimited. 7

Bulk Synthesis of Glass for PLD targets Ideas • OPTIMIZATION OF e AND DIELECTRIC BREAKDOWN FIELD • AF 45: 12% Ba. O. Glass patents from ‘ 60 s: 49% Ba. O (AF) • Dependence of e on Ba. O content • Dependence of breakdown voltage on Ba. O content - - - - - - - - - - - - • Micro-crystallites – increase mechanical strength increase breakdown voltage? or nucleate breakdown? • Flame polishing as a means of improving surface morphology, for both bulk and PLD samples • Neural Network / Genetic Algorithm optimization of Ba. O composition-property relationships Place Proper DISTRIBUTION STATEMENT Here contractors Distribution A: approved authorized for public to DOD release, and USdistribution DOD unlimited. 8

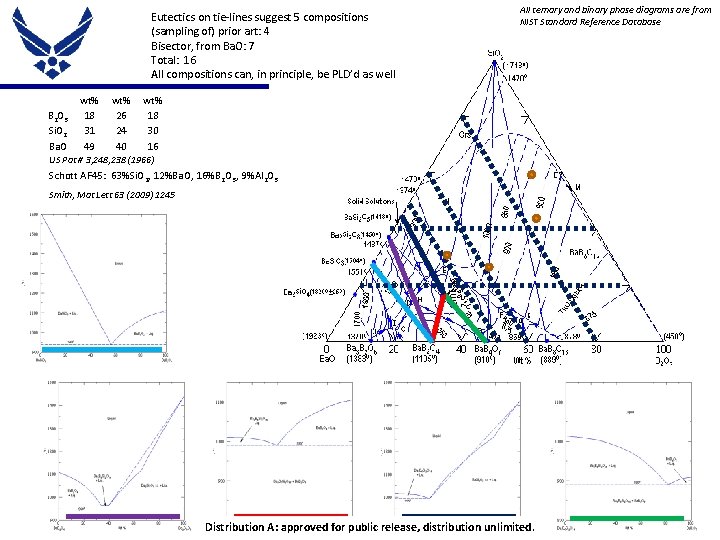
Eutectics on tie-lines suggest 5 compositions (sampling of) prior art: 4 Bisector, from Ba. O: 7 Total: 16 All compositions can, in principle, be PLD’d as well B 2 O 3 Si. O 2 Ba. O wt% 18 31 49 wt% 26 24 40 All ternary and binary phase diagrams are from NIST Standard Reference Database wt% 18 30 16 US Pat # 3, 248, 238 (1966) Schott AF 45: 63%Si. O 2, 12%Ba. O, 16%B 2 O 3, 9%Al 2 O 3 Smith, Mat Lett 63 (2009) 1245 Place Proper DISTRIBUTION STATEMENT Here contractors Distribution A: approved for public release, unlimited. Distribution authorized to DOD and USdistribution DOD 9

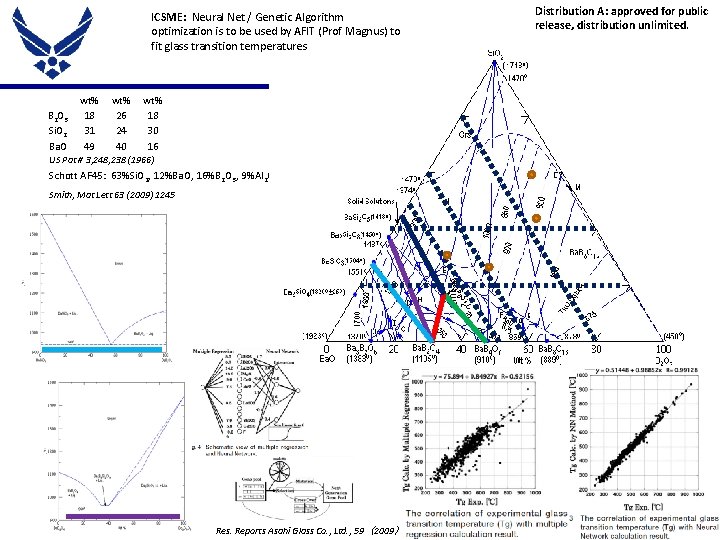
ICSME: Neural Net / Genetic Algorithm optimization is to be used by AFIT (Prof Magnus) to fit glass transition temperatures B 2 O 3 Si. O 2 Ba. O wt% 18 31 49 wt% 26 24 40 Distribution A: approved for public release, distribution unlimited. wt% 18 30 16 US Pat # 3, 248, 238 (1966) Schott AF 45: 63%Si. O 2, 12%Ba. O, 16%B 2 O 3, 9%Al 2 O 3 Smith, Mat Lett 63 (2009) 1245 Place Proper DISTRIBUTION STATEMENT Here Res. Reports Asahi Glass Co. , Ltd. , 59 (2009 ) 10

Synthesized compositions We have chosen to proceed along a bisector of constant boron: silica composition, reaching towards the region of highest barium concentration. The compositions we have synthesized to date are indicated with numbers 7 -10. Barium increases to the left. Proper DISTRIBUTION STATEMENT Here Distribution A: Place approved for public release, distribution unlimited. 11

Samples for pulsed laser deposition targets The numbers in the figure indicate the sample number, referenced to Table 1. For scale, the sample boxes are approximately 1” on a side. All these samples were synthesized in the RX Materials and Manufacturing Directorate. Above is a pellet pressed (non-glass) that has not been sintered. Sintering allows densification and permits the pellet to be used as a viable target for PLD. Pellet was pressed in the RX Materials and Manufacturing Directorate. Proper DISTRIBUTION Here Distribution. Place A: approved for public. STATEMENT release, distribution unlimited. 12

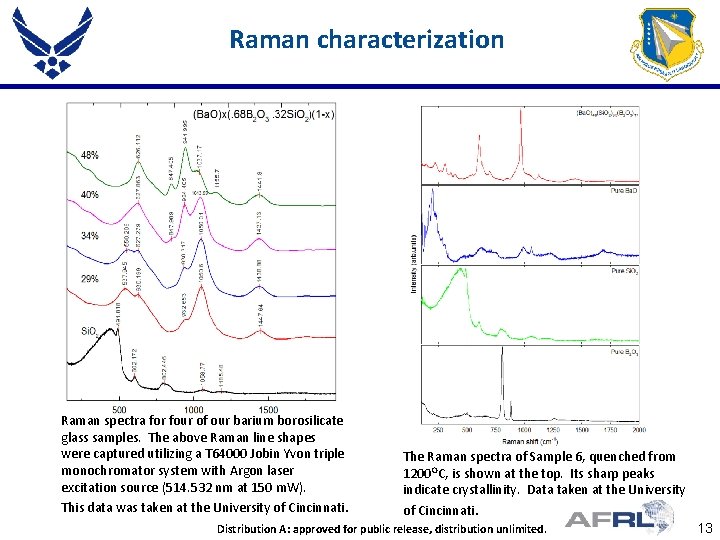
Raman characterization Raman spectra for four of our barium borosilicate glass samples. The above Raman line shapes were captured utilizing a T 64000 Jobin Yvon triple monochromator system with Argon laser excitation source (514. 532 nm at 150 m. W). This data was taken at the University of Cincinnati. The Raman spectra of Sample 6, quenched from 1200 OC, is shown at the top. Its sharp peaks indicate crystallinity. Data taken at the University of Cincinnati. Place Proper DISTRIBUTION STATEMENT Distribution A: approved for public release, Here distribution unlimited. 13

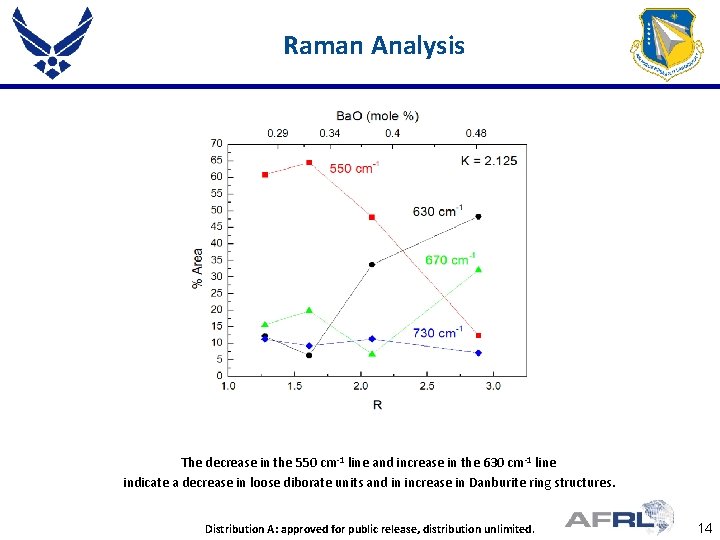
Raman Analysis The decrease in the 550 cm-1 line and increase in the 630 cm-1 line indicate a decrease in loose diborate units and in increase in Danburite ring structures. Place Proper DISTRIBUTION Here Distribution A: approved for public STATEMENT release, distribution unlimited. 14

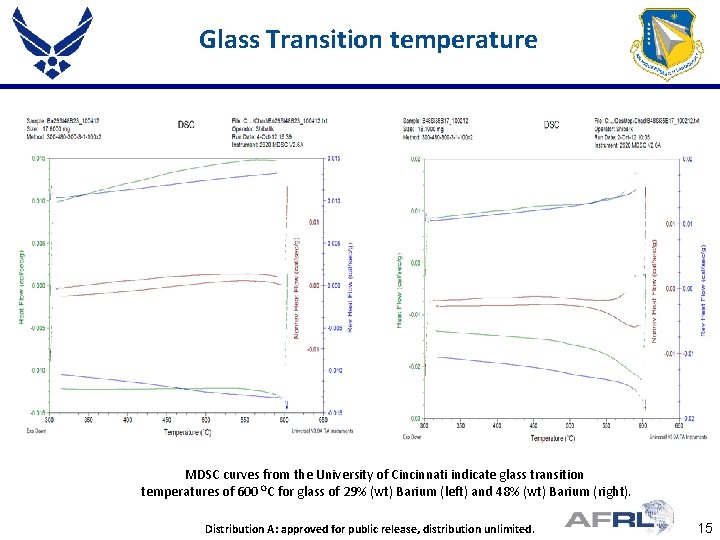
Glass Transition temperature MDSC curves from the University of Cincinnati indicate glass transition temperatures of 600 OC for glass of 29% (wt) Barium (left) and 48% (wt) Barium (right). Place Proper DISTRIBUTION Here Distribution A: approved for public STATEMENT release, distribution unlimited. 15

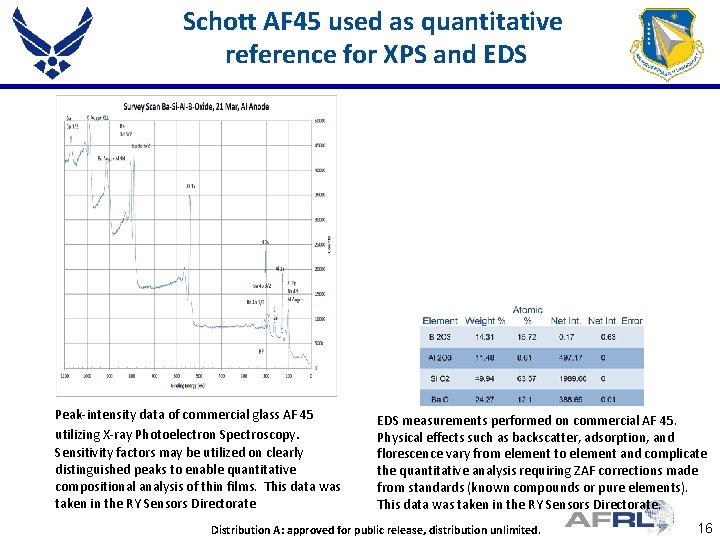
Schott AF 45 used as quantitative reference for XPS and EDS Peak-intensity data of commercial glass AF 45 utilizing X-ray Photoelectron Spectroscopy. Sensitivity factors may be utilized on clearly distinguished peaks to enable quantitative compositional analysis of thin films. This data was taken in the RY Sensors Directorate EDS measurements performed on commercial AF 45. Physical effects such as backscatter, adsorption, and florescence vary from element to element and complicate the quantitative analysis requiring ZAF corrections made from standards (known compounds or pure elements). This data was taken in the RY Sensors Directorate. Proper DISTRIBUTION Here Distribution. Place A: approved for public. STATEMENT release, distribution unlimited. 16

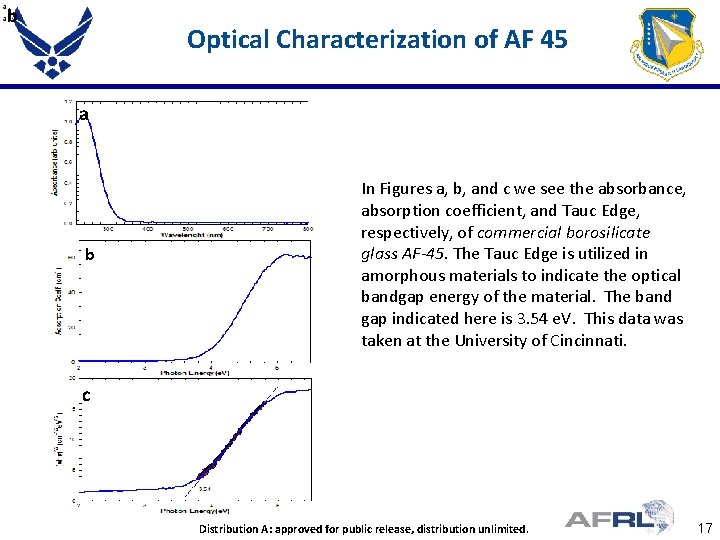
b Optical Characterization of AF 45 a b In Figures a, b, and c we see the absorbance, absorption coefficient, and Tauc Edge, respectively, of commercial borosilicate glass AF-45. The Tauc Edge is utilized in amorphous materials to indicate the optical bandgap energy of the material. The band gap indicated here is 3. 54 e. V. This data was taken at the University of Cincinnati. c Proper DISTRIBUTION STATEMENT Here Distribution A: Place approved for public release, distribution unlimited. Distribution Unlimited 17

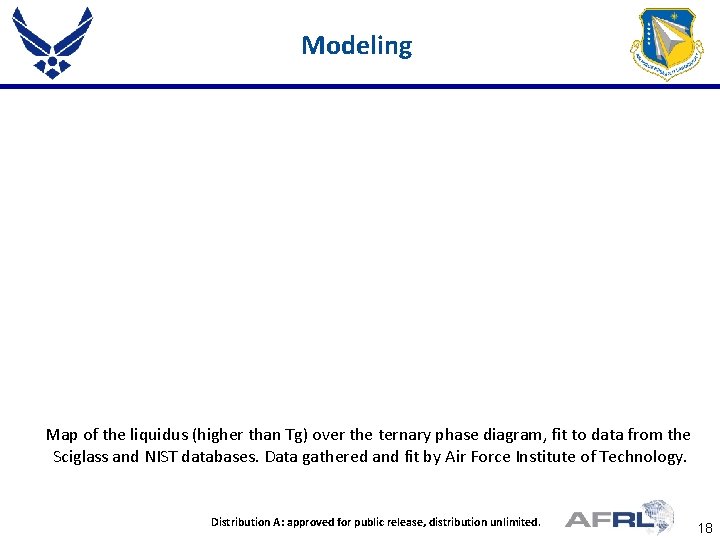
Modeling Map of the liquidus (higher than Tg) over the ternary phase diagram, fit to data from the Sciglass and NIST databases. Data gathered and fit by Air Force Institute of Technology. Distribution. Place A: approved for public release, distribution unlimited. Proper DISTRIBUTION STATEMENT Here 18

Future Work • Continue work on glass modeling and synthesis • Develop targets for pulsed laser deposition • Develop thin layers for simple device characterization • Characterize devices by dielectric, polarization, and breakdown measurements Place Proper DISTRIBUTION STATEMENT Here Distribution A: approved for public release, distribution unlimited. 19

Abstracts submitted for presentation • Molecular Structure of the (Ba. O)x(. 68 Si. O 2. 32 B 2 O 3)1 -x amorphous system. PACRIM 10, San Diego 2 June 2013. Proper DISTRIBUTION Here Distribution. Place A: approved for public. STATEMENT release, distribution unlimited. Unlimited Distr 20

The Team • AFRL/RX Ed Stutz- PI Jonathan Goldstein- Co PI, Glass synthesis • AFRL/RY Chad Holbrook- Glass synthesis, • AFIT Amy Magnus- Glass calculations Adam Brandt- EPR-ENDORE measurements • University of Cincinnati P. Boolchand- Material characterization • University of Dayton Research Institute Gregory Kozlowski - PLD growth Steve Smith- Dielectric characterization Gerry Landis- single layer device fabrication Howard Smith- microscopy techniques • Penn State Mike Lanagan- Self healing electrode studies • Wright State Jason Anders Other collaborators • RD Susan Heidger - Requirements • RZ Jennifer De. Cerbo - voltage breakdown facility • RX Michael Durstock - Composite dielectrics • On-site help - Steve Fairchild - Tyson Back - Terry Murray Place Proper DISTRIBUTION STATEMENT Here contractors Distribution A: approved for public distribution unlimited. Distribution authorized torelease, DOD and US DOD 21