Investigation 7 B The Geometry of Molecules Structural

Investigation 7 B The Geometry of Molecules
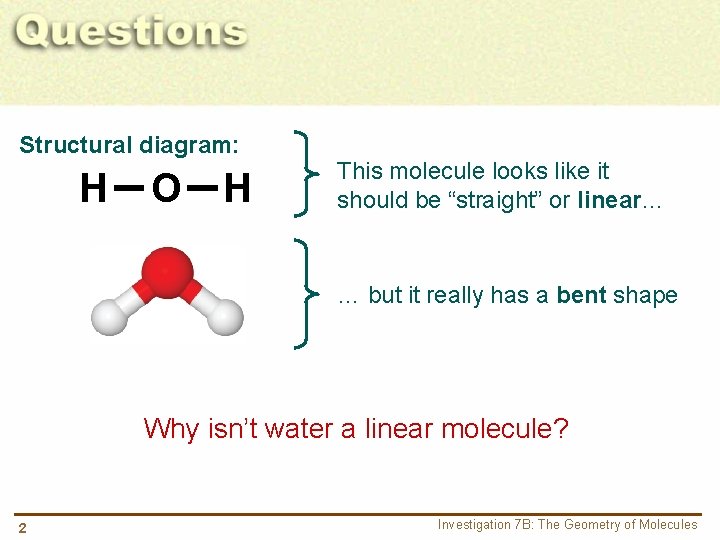
Structural diagram: H O H This molecule looks like it should be “straight” or linear… … but it really has a bent shape Why isn’t water a linear molecule? 2 Investigation 7 B: The Geometry of Molecules
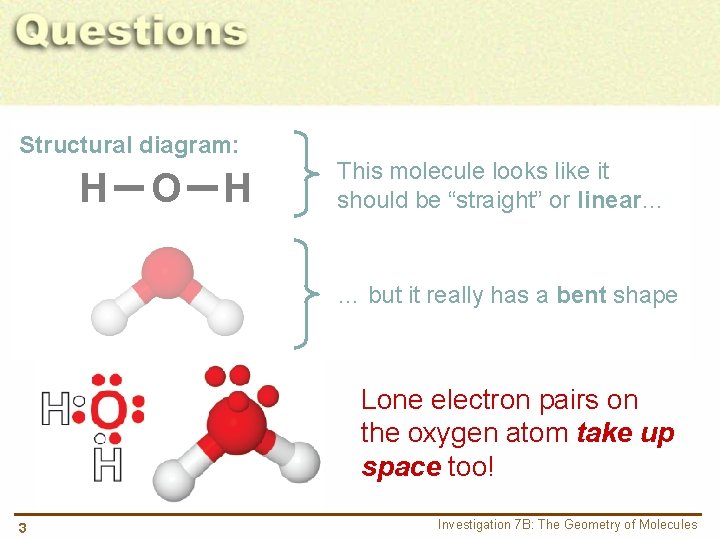
Structural diagram: H O H This molecule looks like it should be “straight” or linear… … but it really has a bent shape Lone electron pairs on the oxygen atom take up space too! 3 Investigation 7 B: The Geometry of Molecules
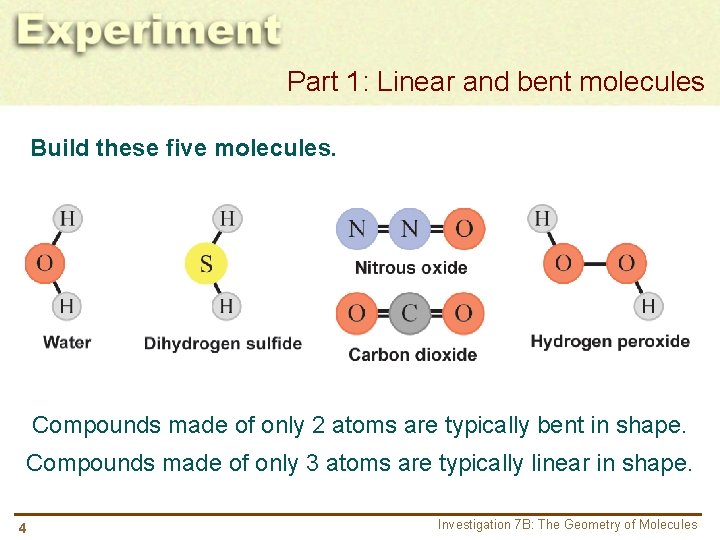
Part 1: Linear and bent molecules Build these five molecules. Compounds made of only 2 atoms are typically bent in shape. Compounds made of only 3 atoms are typically linear in shape. 4 Investigation 7 B: The Geometry of Molecules

Part 1: Linear and bent molecules a. Name two other elements which would likely form a bent molecule when bound with two hydrogen atoms. b. Create two other linear molecules. What elements do they contain? 5 Investigation 7 B: The Geometry of Molecules
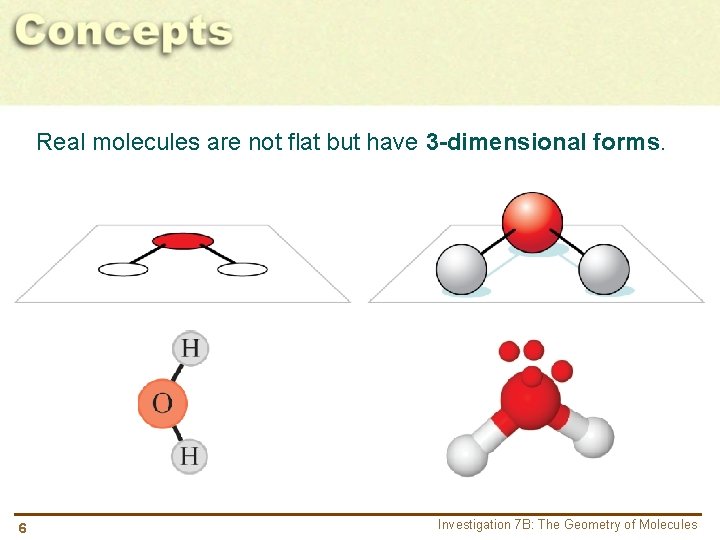
Real molecules are not flat but have 3 -dimensional forms. 6 Investigation 7 B: The Geometry of Molecules
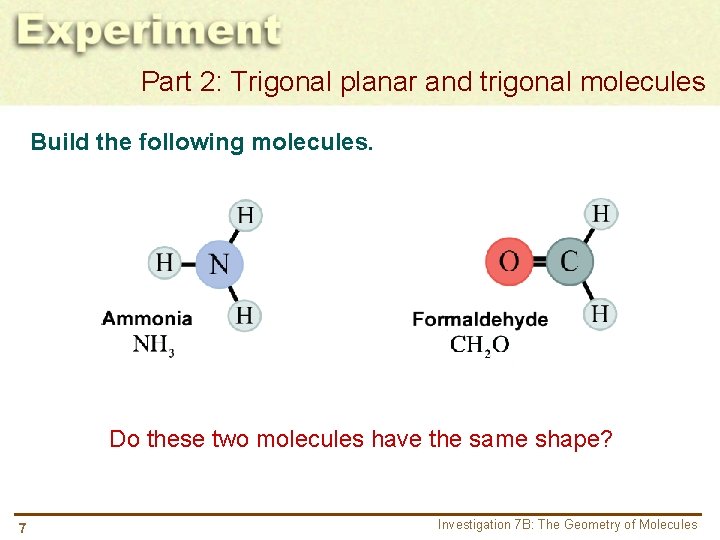
Part 2: Trigonal planar and trigonal molecules Build the following molecules. Do these two molecules have the same shape? 7 Investigation 7 B: The Geometry of Molecules
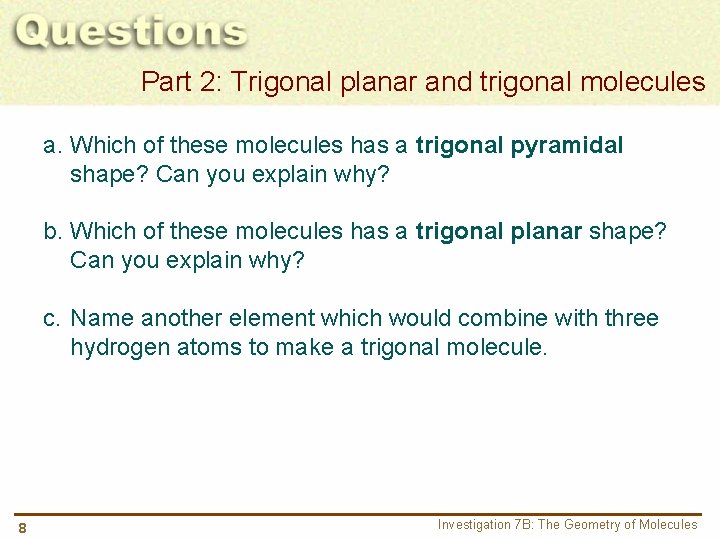
Part 2: Trigonal planar and trigonal molecules a. Which of these molecules has a trigonal pyramidal shape? Can you explain why? b. Which of these molecules has a trigonal planar shape? Can you explain why? c. Name another element which would combine with three hydrogen atoms to make a trigonal molecule. 8 Investigation 7 B: The Geometry of Molecules
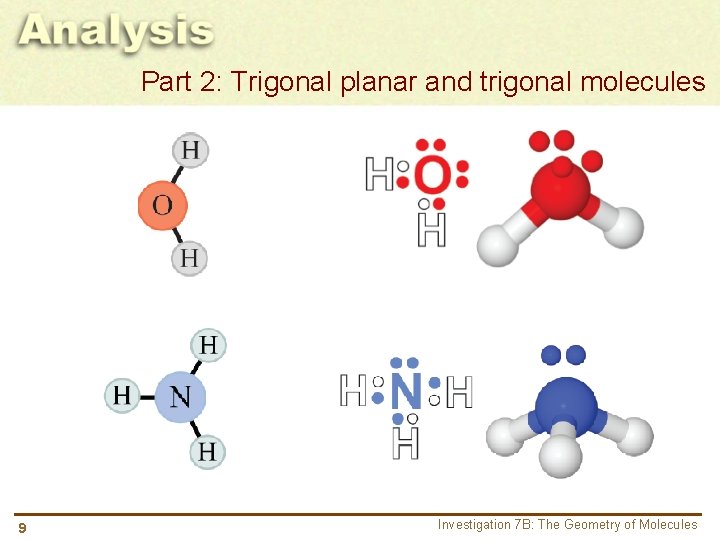
Part 2: Trigonal planar and trigonal molecules 9 Investigation 7 B: The Geometry of Molecules
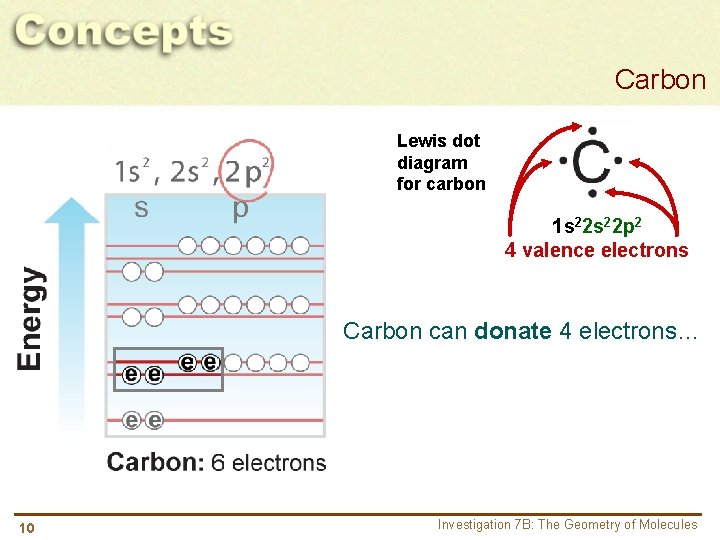
Carbon Lewis dot diagram for carbon 1 s 22 p 2 4 valence electrons Carbon can donate 4 electrons… 10 Investigation 7 B: The Geometry of Molecules
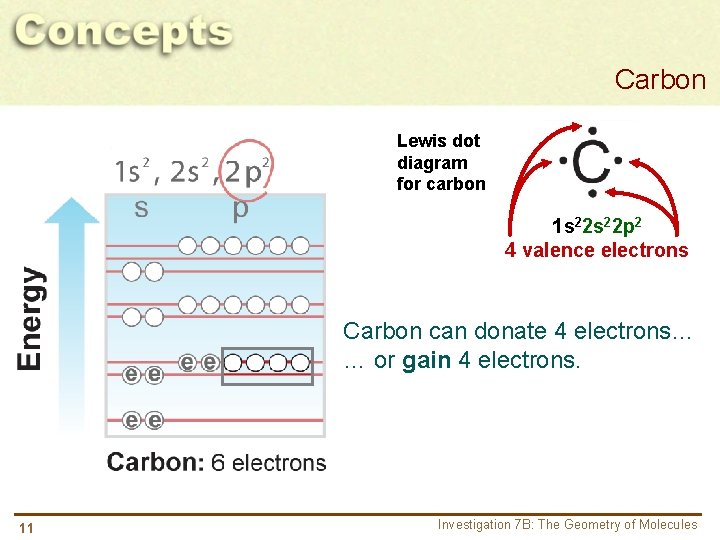
Carbon Lewis dot diagram for carbon 1 s 22 p 2 4 valence electrons Carbon can donate 4 electrons… … or gain 4 electrons. 11 Investigation 7 B: The Geometry of Molecules
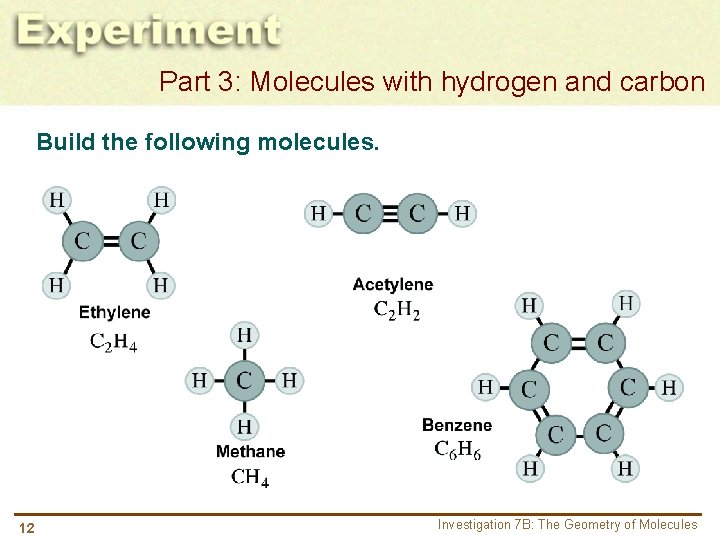
Part 3: Molecules with hydrogen and carbon Build the following molecules. 12 Investigation 7 B: The Geometry of Molecules
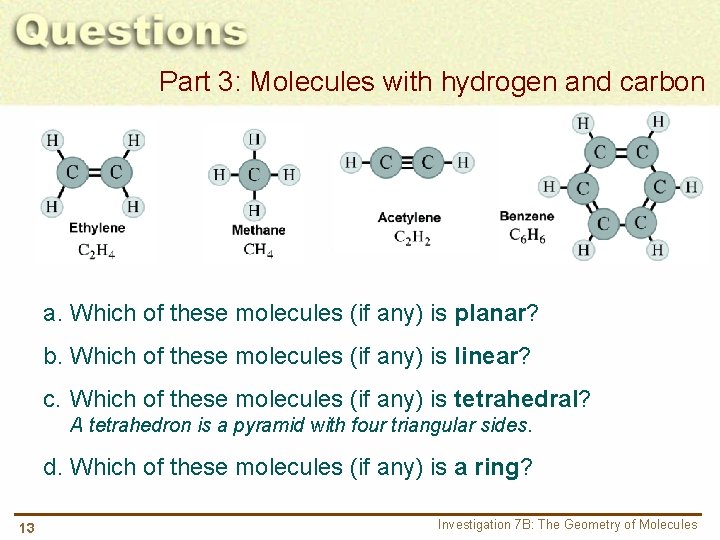
Part 3: Molecules with hydrogen and carbon a. Which of these molecules (if any) is planar? b. Which of these molecules (if any) is linear? c. Which of these molecules (if any) is tetrahedral? A tetrahedron is a pyramid with four triangular sides. d. Which of these molecules (if any) is a ring? 13 Investigation 7 B: The Geometry of Molecules
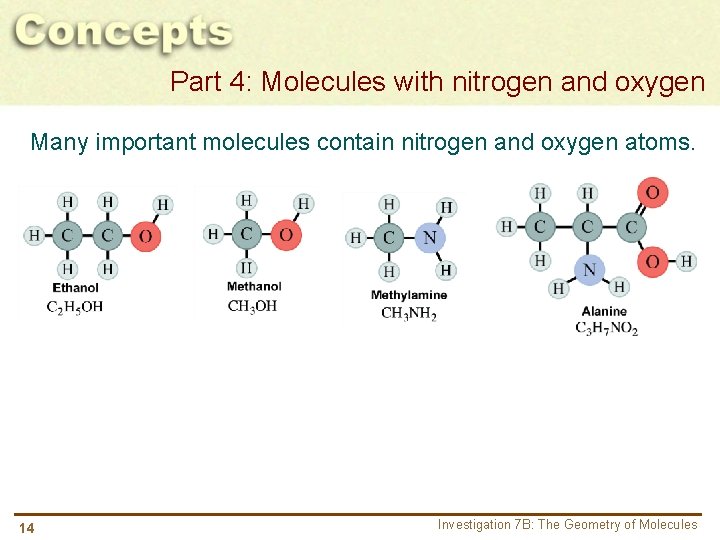
Part 4: Molecules with nitrogen and oxygen Many important molecules contain nitrogen and oxygen atoms. 14 Investigation 7 B: The Geometry of Molecules
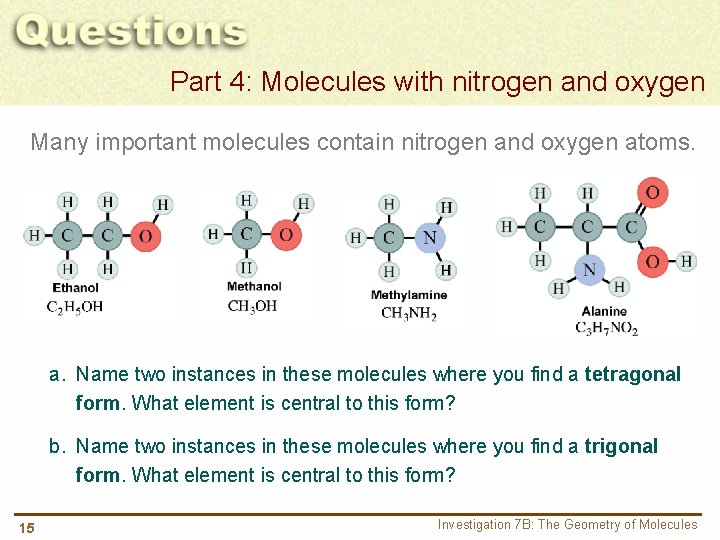
Part 4: Molecules with nitrogen and oxygen Many important molecules contain nitrogen and oxygen atoms. a. Name two instances in these molecules where you find a tetragonal form. What element is central to this form? b. Name two instances in these molecules where you find a trigonal form. What element is central to this form? 15 Investigation 7 B: The Geometry of Molecules
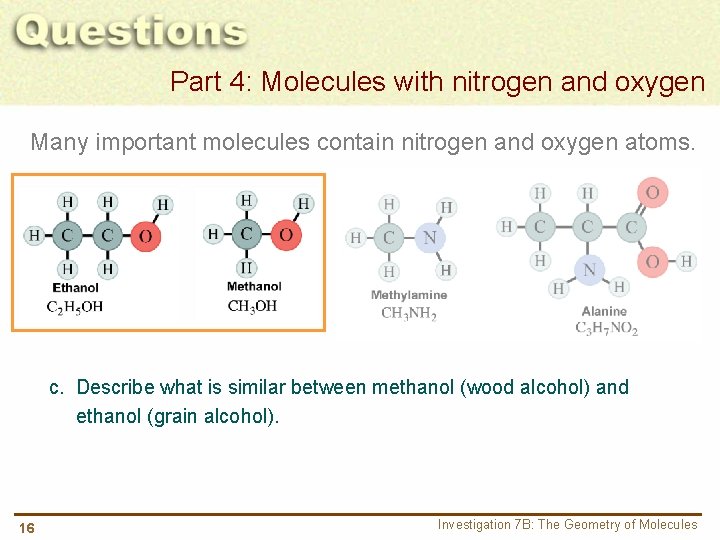
Part 4: Molecules with nitrogen and oxygen Many important molecules contain nitrogen and oxygen atoms. c. Describe what is similar between methanol (wood alcohol) and ethanol (grain alcohol). 16 Investigation 7 B: The Geometry of Molecules
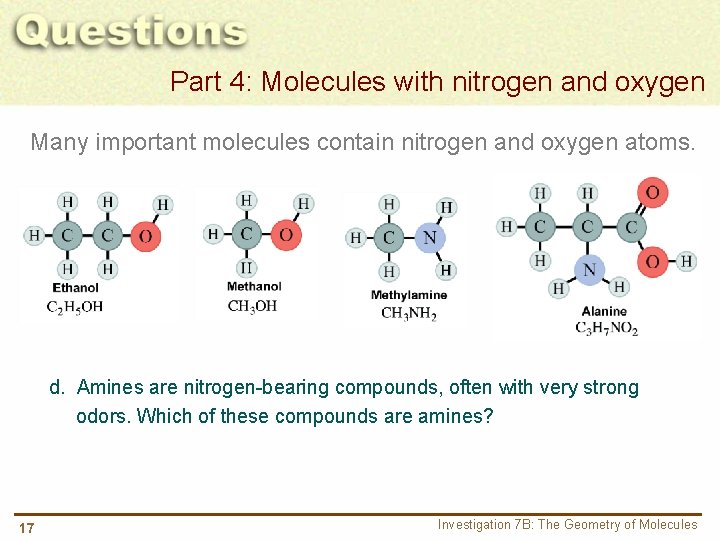
Part 4: Molecules with nitrogen and oxygen Many important molecules contain nitrogen and oxygen atoms. d. Amines are nitrogen-bearing compounds, often with very strong odors. Which of these compounds are amines? 17 Investigation 7 B: The Geometry of Molecules
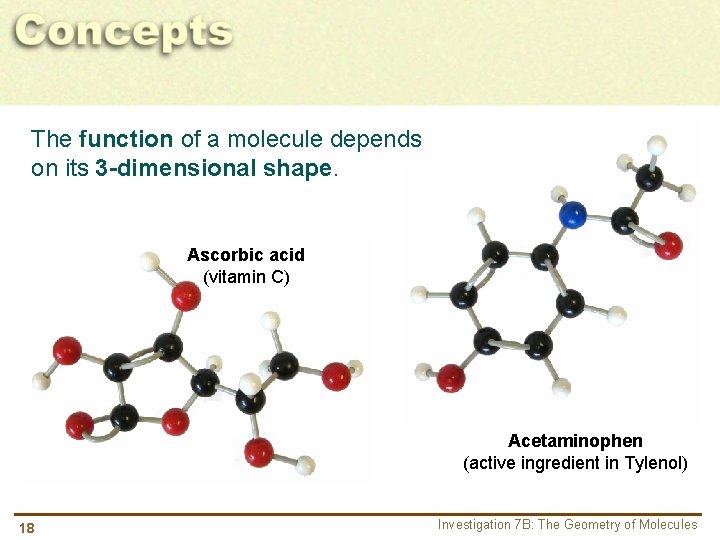
The function of a molecule depends on its 3 -dimensional shape. Ascorbic acid (vitamin C) Acetaminophen (active ingredient in Tylenol) 18 Investigation 7 B: The Geometry of Molecules
- Slides: 18