Inventory Management Chapter 13 Inventory Management n An




















- Slides: 24

Inventory Management Chapter 13

Inventory Management n An inventory is a listing of all the goods or items that a business will use in its normal operation. n The goal of inventory management is to have drugs available (and usable) when they are needed. n Example: during flu season, the pharmacy might carry extra antibiotics, or cough medication.

Inventory Management n In some cases inventory management may be the technician’s primary responsibility. n The technician would be the “Pharmaceutical Buyer. ” n This would also require the technician to check all expiration dates and return all expired drugs according to the manufacturer’s requirements.

Formulary n Formulary: a list of medications that are approved for use. n Many hospitals, HMOs, insurers and other health care systems maintain a list of medications (formulary) that is for use within that system.

Formulary n An open formulary is one that allows the purchase of any medication that is prescribed. n The patient’s copay may vary depending upon the medication. n Generic drugs are usually first line therapy.

Formulary n A closed formulary is a limited list of medications. n A physician must receive permission to use a medication that is not on the list (prior authorization). n Generic drugs are usually the first line of therapy.

Legal Requirements n The DEA regulates the distribution of controlled substances, and has various inventory, record keeping and ordering requirements. n Schedule II substances must be: n Stocked separately in a secure place n Require special order forms for reordering n Inventory must be continuously monitored & documented (perpetual inventory)

Inventory Systems n A pharmaceutical inventory system is able to track inventory, forecast needs, and generate reorders to maintain adequate supplies. n Perpetual Inventory: maintains a continuous record of every item in the inventory so that it always shows the stock on hand.

Inventory Terms n Turnover: the rate at which inventory is used; it is generally expressed in the number of days. n Point of Sale System (POS): an inventory system which the item is deducted from inventory as it is sold or dispensed. n Reorder Points: minimum and maximum stock levels which determine when a reorder is placed and for what quantity.

Computers & Inventory n Computer inventory systems automatically adjust inventory & generate orders based on maintaining a set inventory levels. n Computers maintain a database; a collection of information structured so specific information can be retrieved and used. n It is important to enter information into the computer correctly to ensure quality inventory reporting. (Garbage in=Garbage

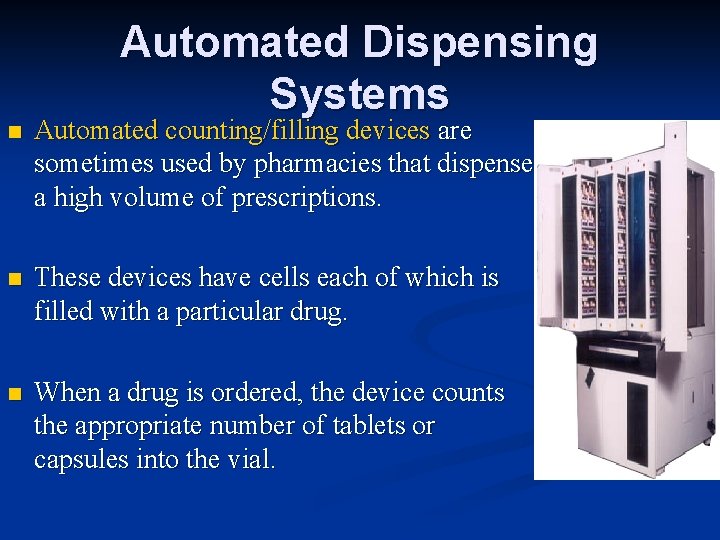
Automated Dispensing Systems n Automated counting/filling devices are sometimes used by pharmacies that dispense a high volume of prescriptions. n These devices have cells each of which is filled with a particular drug. n When a drug is ordered, the device counts the appropriate number of tablets or capsules into the vial.

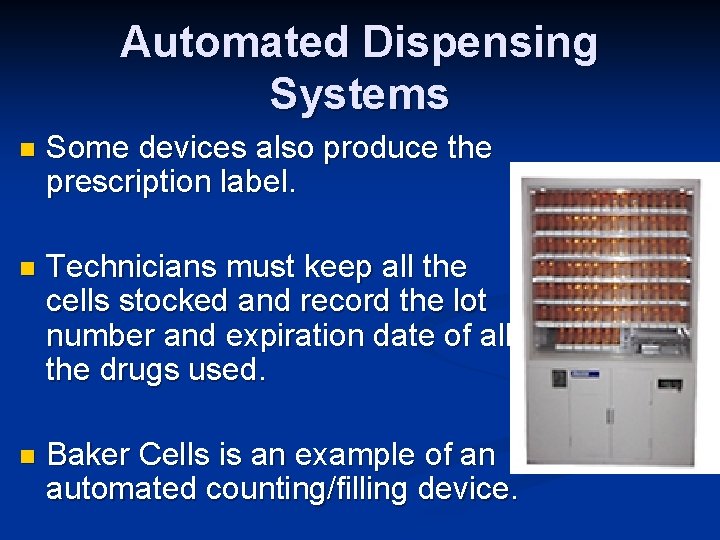
Automated Dispensing Systems n Some devices also produce the prescription label. n Technicians must keep all the cells stocked and record the lot number and expiration date of all the drugs used. n Baker Cells is an example of an automated counting/filling device.

Automated Dispensing Systems n Automated point-of-use storage systems are for making floor stock items available to nurses in the hospital setting. n Storage stations are located throughout the facility and connected to the server and linked to the hospital’s billing and materials management system.

Automated Delivery Systems n The system tracks inventory, keeps a record of what drugs or supplies were taken by which nurse for which patient. n Pyxis Supply Station is an example of an automated pointof-use supply station.

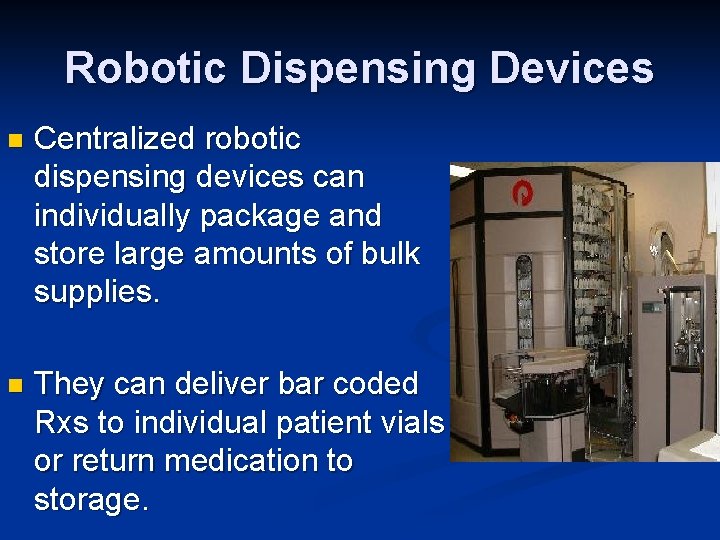
Robotic Dispensing Devices n Centralized robotic dispensing devices can individually package and store large amounts of bulk supplies. n They can deliver bar coded Rxs to individual patient vials or return medication to storage.

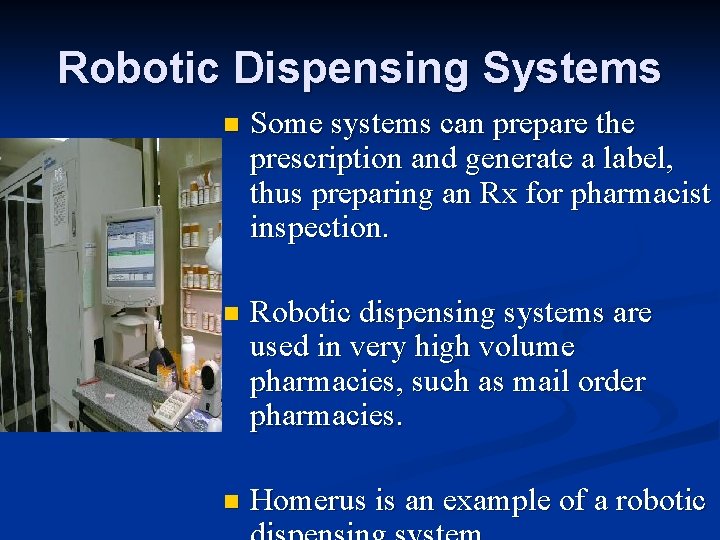
Robotic Dispensing Systems n Some systems can prepare the prescription and generate a label, thus preparing an Rx for pharmacist inspection. n Robotic dispensing systems are used in very high volume pharmacies, such as mail order pharmacies. n Homerus is an example of a robotic

Key Computer Considerations n Manual Checking – Always double check! Data entry problems can be easily corrected if caught early. This eliminates stock problems, pricing & billing errors. n System Maintenance – It is important to learn the care, maintenance & back up requirements of your workplace system to avoid data loss or corruption.

Ordering n Ordering systems involve automated and manual activities. (see page 320) n It is very important to check the order being sent and the order that is received. n Ordering issues should be resolved immediately.

Ordering n Three-quarters of the pharmaceutical manufacturer’s sales are to drug wholesalers. n These wholesalers then sell their inventory to pharmacies, hospitals and other dispensers. n Drugs can also be purchased directly from the manufacturer.

Receiving n Accuracy is essential in checking in the medications received from suppliers. n Drugs that have been incorrectly picked, received damaged, are outdated or missing entirely must be reported immediately. n Discrepancies must be resolved with the vendor immediately.

Stocking & Storing n Most medications are received in bulk “stock bottles” that carry FDA required information on the label. n Some medications are packaged in individual doses called “unit-dose” packaging. n Regardless of packaging, all drugs must be stored according to the manufacturer’s specifications.

Stocking & Storing n Most drugs are kept in a fairly constant room temperature of 59º-86º F. n Only when the manufacturer specifies, should you store drugs less than room temperature. n The temperature of refrigeration should generally be 40º - 42º F.

Quarantined Stock n Stock that has expired, been damaged, recalled, or otherwise targeted for return or disposal must be segregated and clearly marked to avoid contamination, and/or mix-up with the good stock.

Chapter 13 n Read Chapter 13 n Review Forms pages 322 -323 n Review Key Concepts n Review Self-Test