Invasive and Respiratory Infectious Disease Updates ELC EPIDEMIOLOGY






![Amebic Meningitis/Encephalitis (GAE [Balamuthia, Acanthamoeba], PAM [Naegleria fowleri], & other) Need to investigate fully Amebic Meningitis/Encephalitis (GAE [Balamuthia, Acanthamoeba], PAM [Naegleria fowleri], & other) Need to investigate fully](https://slidetodoc.com/presentation_image_h2/05f226cc7628868bb6481ff51e5ce95b/image-7.jpg)













- Slides: 23

Invasive and Respiratory Infectious Disease Updates ELC EPIDEMIOLOGY WORKSHOP IRID/VPD BREAKOUT SESSION FEBRUARY 25, 2016 Lesley Brannan, MPH IRID Team Leader

IRID Team Bob Johnathan Lesley Amy ? ? ?

IRID Diseases Notifiable Legionellosis Invasive meningococcal infection Invasive streptococcal diseases Invasive Group A Streptococcus (S. pyogenes) Invasive Group B Streptococcus (S. agalactiae) Invasive Streptococcus pneumoniae Novel coronavirus Novel/variant influenza A Influenza-associated pediatric mortality Amebic meningitis/encephalitis, including Primary Amebic Meningoencephalitis (PAM) Non-notifiable Influenza Respiratory syncytial virus (RSV)

Legionellosis Investigation Guidelines Updates Basic epi: Note about incubation period for outbreaks, added severity info for disease types (p. 155) Case Investigation checklist: clarified investigation forms to use for cases, updated web links, added recommendations for documentation to determine onset date, slight reordering of section (p. 157 -158) Prevention and control: updated water birth guidelines (pp. 159 -161), added indications for Legionella testing (p. 159), added and updated water maintenance bullet (p. 159) Managing Special Situations: clearly defined sections for single cases vs. multiple cases; added water system maintenance guidance link; updated web links; added training video link for environmental assessment; updated ASHRAE standard; ask labs to retain Legionella isolates (p. 161 -169) Environmental Sampling and Testing: extensive changes (pp. 173 -177) Additional Resources: basically, new section (pp. 177 -178)

Legionellosis – New and Coming Soon-ish CDC new resources Legionellosis investigation toolkits: http: //www. cdc. gov/legionella/health-depts/index. html CDC environmental investigation videos: http: //www. cdc. gov/legionella/videos. html On the horizon Near: Improved/expanded investigation form Far: NBS changes

Amebic Meningitis/Encephalitis (including PAM) – Guidance Updates Case criteria – rewording of description only, to align with national EAIDB Investigation Guidelines Added transmission through organ transplantation (pp. 13 -14) Edited incubation period, illness duration (p. 14) Added ritual nasal rinsing, ablution to prevention section (p. 18) Clarified that only waterborne outbreaks need to be reported in NORS (p. 19) Lab section: Added required forms, added telediagnosis info (CDC), updated shipping and contact information (pp. 19 -22)
![Amebic MeningitisEncephalitis GAE Balamuthia Acanthamoeba PAM Naegleria fowleri other Need to investigate fully Amebic Meningitis/Encephalitis (GAE [Balamuthia, Acanthamoeba], PAM [Naegleria fowleri], & other) Need to investigate fully](https://slidetodoc.com/presentation_image_h2/05f226cc7628868bb6481ff51e5ce95b/image-7.jpg)
Amebic Meningitis/Encephalitis (GAE [Balamuthia, Acanthamoeba], PAM [Naegleria fowleri], & other) Need to investigate fully – Complete “Free-Living Ameba Case Report” form Get detailed info Get all medical records Interview family/surrogate for exposures Determine whether organs/corneas were donated Miltefosine update Summer safety intern – PAM, Crypto Press release bullets Educational material – pediatricians, camps, parks, etc.

Meningococcal Case Definition Updates Case definition change Removed “clinically compatible” requirement Moved two formerly probable categories to suspect GNDC clarifying statement 2016 case definition (effective January 1, 2016): Confirmed: A case that is laboratory confirmed Probable: A case that has one of the following: N. meningitidis antigen detection by immunohistochemistry (IHC) on formalin-fixed tissue N. meningitidis antigen detection by latex agglutination of CSF Suspect: A case that has one of the following: Clinical purpura fulminans in the absence of a positive blood culture Gram-negative diplococci, not yet identified, isolated from a normally sterile site (e. g. , blood or CSF )

Meningococcal – More Updates Investigation, Reporting, NBS: Case investigation, contact tracing, contact prophylaxis for C, P, and S cases Enter C, P, and S cases in NBS and submit notifications Only C and P cases counted in official numbers Meningitis is not the only clinical presentation (p. 210) Most common in TX: meningitis (48%) bacteremia/septicemia pneumonia (3%) (34%)

Invasive Meningococcal Infection – Investigation Guidelines and Form EAIDB Investigation Guidelines updates Added clinical manifestations and occurrence in TX (p. 210) Clarifications throughout for meningococcal disease vs. meningitis Reporting and Data Entry Requirements: added instructions for suspect cases (p. 218) Added request for nonviable isolates and sterile sites specimens when isolates are not available (p. 219) Updated Invasive Meningococcal Infection: Case Status Classification flowchart (p. 222) On the horizon Investigation form updates, including 3 new sexual contacts/history questions

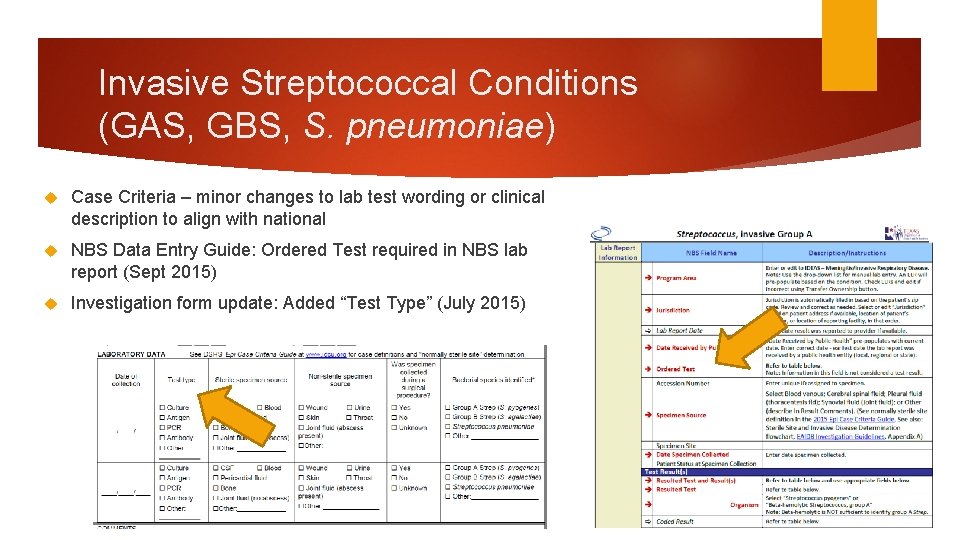
Invasive Streptococcal Conditions (GAS, GBS, S. pneumoniae) Case Criteria – minor changes to lab test wording or clinical description to align with national NBS Data Entry Guide: Ordered Test required in NBS lab report (Sept 2015) Investigation form update: Added “Test Type” (July 2015)

Invasive Streps – Investigation Guidelines and NBS EAIDB Investigation Guidelines: Emphasized need to collect enough information to meet case definition (pp. 323, 329, 333) EAIDB Investigation Guidelines flowcharts Invasive Streptococcal Infection: Case Status Classification (p. 386) – emphasized that alpha and beta hemolysis is not the same as the group (e. g. , Streptococcus, alpha hemolytic ≠ Group A Strep) Sterile Site and Invasive Disease Determination (pp. 383 -384) – cord blood and respiratory specimens are not sterile sites, joint fluid might be a sterile site NBS: If a lab report is not associated with the investigation, put enough information in the investigation comments so we can tell it meets case definition (i. e. , test type, test result, specimen source, and clinical syndrome [if necrotizing fasciitis or toxic shock syndrome with specimen from non-sterile site]) We will reject 2016 cases if there is no lab information(i. e. , test type, test result, specimen source), or the provided lab data do not meet case definition.


Immunization Grant Requirements (meningococcal, S. pneumoniae) You have 30 days from time of report to HD until case is finished (investigated, reported in NBS, etc. ) Obtain vaccination status Sources: patient/parent/surrogate, PCP, hospital/reporting facility, Imm. Trac, employer, school/daycare Children: a record exists Adults: try your best Get serogroup – send in those isolates to DSHS Austin Lab! Required for Neisseria meningitidis Voluntary for S. pneumoniae (isolates from sterile sites, kids <5 yo) Patient outcome Follow patient until discharge

Novel Coronavirus/MERS EAIDB Investigation Guidelines changes Updated PUI definition – fever note; removed Republic of Korea (p. 244) For anyone tested for MERS-Co. V, PUI form must be completed and submitted to DSHS within 48 hours of testing (p. 245) Prevention and Control Measures: specific recommendations provided throughout section (pp. 247 -262) New: Air and Ground Medical Transport section (p. 256) – REPLACE w/handout Laboratory Procedures: attempted to clarify when serum should be collected for r. RT-PCR testing at a state or local PHL (more common) vs. PCR testing at CDC (less common) (pp. 266 -267)

Novel Coronavirus/MERS PUI Criteria Updates from CDC Deleted reference to the Republic of Korea More than two incubation periods have passed since the last MERS case was reported from the Republic of Korea. Revised the MERS Patient Under Investigation (PUI) Short Form. Added footnote to PUI Guidance clarifying that fever may not be present in some patients, such as those who are very young, elderly, immunosuppressed, or taking certain medications. Clinical judgement should be used to guide testing of patients in such situations. Revised document title to “Interim Patient Under Investigation (PUI) Guidance and Case Definitions for MERS” from “Case Definitions” to highlight the current clinical features and epidemiologic risks that guide testing and decisions for a patient under investigation, rather than using a more absolute case definition.

Novel/Variant Influenza EAIDB Investigation Guidelines: changed Case Under Investigation definition and footnotes for “Novel Influenza A Viruses with the Potential to Cause Severe Disease in Humans” (p. 140)

Influenza-associated Pediatric Mortality Updates Get flu vaccine history (often missing) EAIDB is routinely receiving vital stats data If pediatric influenza deaths are found you will be asked to investigate

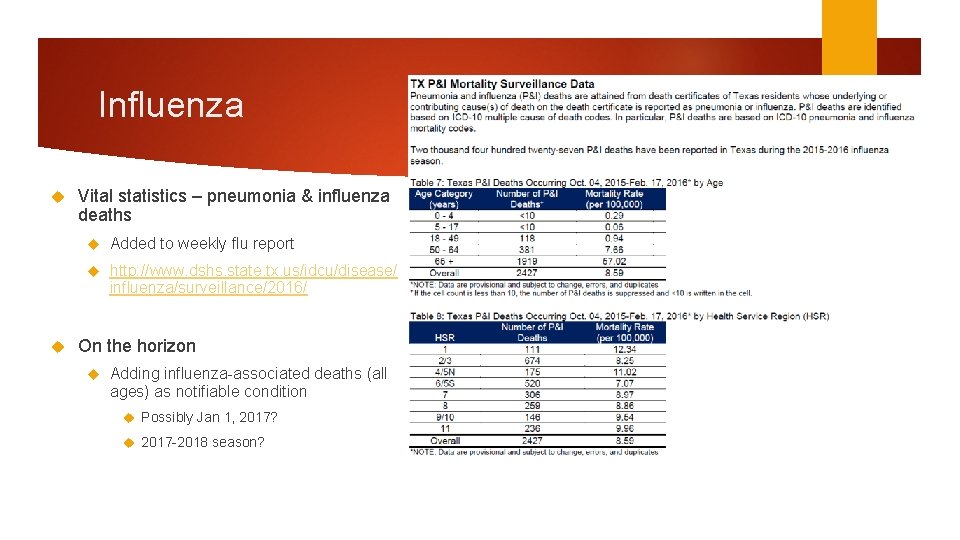
Influenza Vital statistics – pneumonia & influenza deaths Added to weekly flu report http: //www. dshs. state. tx. us/idcu/disease/ influenza/surveillance/2016/ On the horizon Adding influenza-associated deaths (all ages) as notifiable condition Possibly Jan 1, 2017? 2017 -2018 season?

Influenza Surveillance Workshop (influenza, MERS/novel coronavirus, respiratory viruses) – Summer 2016 Any influenza, influenza-like illness, or respiratory virus (non-VPD) questions? Contact us at flutexas@dshs. state. tx. us

ILINet US Outpatient Influenza-like Illness Surveillance Network (ILINet) Voluntary nationwide network of outpatient providers who report weekly on number of patients seen with influenza-like illness (ILI) by age group, and total patients seen for any reason Can calculate % ILI definition: fever ≥ 100°F plus cough and/or sore throat CDC recruitment target: 1 provider per 250, 000 population Providers urgently needed in Regions 1 (Lubbock, Potter, Hale) and 4 (Smith, Gregg, Bowie) Provider participation takes about 20 minutes/week Contact Robert Russin to enroll providers Only non-lab flu surveillance system to “detect” the start of the 2009 pandemic To log in to the online system to see data from Texas, contact Robert Russin

NREVSS National Respiratory and Enteric Virus Surveillance System (NREVSS) – CDC online system Weekly data (# total tests, # tests positive) voluntarily entered by hospital laboratories Viral data captured (any/all of the following): influenza, parainfluenza, RSV, human metapneumovirus, rhinovirus, enterovirus, adenovirus (respiratory and enteric), seasonal coronaviruses, rotavirus Labs urgently needed in Regions 2, 4, 9, 10, 1 (or anywhere there is an interested lab) Contact Johnathan to enroll new labs

Respiratory Syncytial Virus (RSV) Trends in RSV lab detections inform Texas Medicaid office on approval dates for palivizumab (Ig monoclonal Ab) Monthly injections recommended for children with certain risk factors for RSV – see 2015 Red Book for list or http: //pediatrics. aappublications. org/content/134/2/415. full DSHS Austin makes weekly graphs of RSV test data from NREVSS Testing trends PCR testing is becoming more common but no season threshold exists Antigen testing is becoming less common Stay tuned for changes to graphs, etc.
 Chapter 26 infectious disease prevention and control
Chapter 26 infectious disease prevention and control Invasive meningococcal disease
Invasive meningococcal disease Infectious disease quality controls
Infectious disease quality controls Stages of infection
Stages of infection Stages of infectious disease
Stages of infectious disease Infectious disease board review
Infectious disease board review Infectious disease
Infectious disease Hennepin county infectious disease manual
Hennepin county infectious disease manual Smallest infectious agent
Smallest infectious agent Respiratory zone vs conducting zone
Respiratory zone vs conducting zone Fluid cleanliness system
Fluid cleanliness system Elc curriculum
Elc curriculum Elc650
Elc650 Uga elc
Uga elc Elc palm beach
Elc palm beach Nitnem banis timings
Nitnem banis timings Generation z
Generation z Elc 200
Elc 200 Elc.polyu.edu.hk ielts
Elc.polyu.edu.hk ielts Bharathi viswanathan
Bharathi viswanathan Disease respiratory
Disease respiratory Certain infectious and parasitic diseases
Certain infectious and parasitic diseases Poisonous and infectious material symbol
Poisonous and infectious material symbol Difference between descriptive and analytic epidemiology
Difference between descriptive and analytic epidemiology