Invasion and Metastasis Invasion and metastasis the major













































- Slides: 79

Invasion and Metastasis

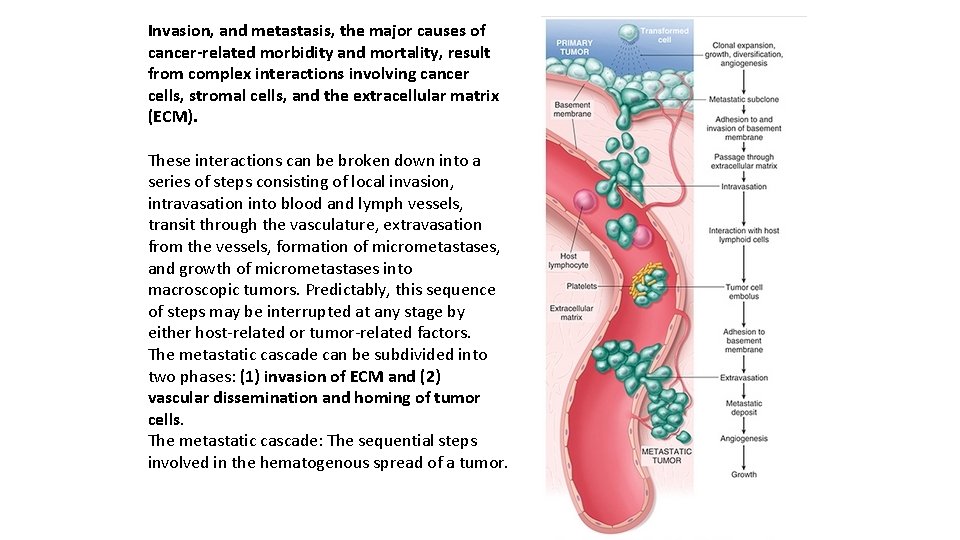
Invasion, and metastasis, the major causes of cancer-related morbidity and mortality, result from complex interactions involving cancer cells, stromal cells, and the extracellular matrix (ECM). These interactions can be broken down into a series of steps consisting of local invasion, intravasation into blood and lymph vessels, transit through the vasculature, extravasation from the vessels, formation of micrometastases, and growth of micrometastases into macroscopic tumors. Predictably, this sequence of steps may be interrupted at any stage by either host-related or tumor-related factors. The metastatic cascade can be subdivided into two phases: (1) invasion of ECM and (2) vascular dissemination and homing of tumor cells. The metastatic cascade: The sequential steps involved in the hematogenous spread of a tumor.

Invasion of Extracellular Matrix Human tissues are organized into a series of compartments separated from each other by two types of ECM: basement membranes and interstitial connective tissue. Although organized differently, each type of ECM is composed of collagens, glycoproteins, and proteoglycans. Tumor cells must interact with the ECM at several stages in the metastatic cascade. A carcinoma first must breach the underlying basement membrane, then traverse the interstitial connective tissue, and ultimately gain access to the circulation by penetrating the vascular basement membrane. This process is repeated in reverse when tumor cell emboli extravasate at a distant site. Invasion of the ECM initiates the metastatic cascade and is an active process that can be resolved into several sequential steps.

Loosening of intercellular connections between tumor cells E-cadherins act as intercellular glues, and their cytoplasmic portions bind to β-catenin. Adjacent E-cadherin molecules keep the cells together; in addition, as discussed earlier, E-cadherin can transmit anti-growth signals by sequestering β-catenin. E-cadherin function is lost in almost all epithelial cancers, either by mutational inactivation of E-cadherin genes, activation of βcatenin genes, or inappropriate expression of the SNAIL and TWIST transcription factors, which suppress E-cadherin expression.


Local degradation of the basement membrane and interstitial connective tissue Tumor cells may either secrete proteolytic enzymes themselves or induce stromal cells (e. g. , fibroblasts and inflammatory cells) to elaborate proteases. Multiple different proteases, such as matrix metalloproteinases (MMPs), cathepsin D, and urokinase plasminogen activator, have been implicated in tumor cell invasion. MMPs regulate tumor invasion not only by remodeling insoluble components of the basement membrane and interstitial matrix but also by releasing ECM-sequestered growth factors, which have chemotactic, angiogenic, and growth-promoting effects. For example, MMP-9 is a gelatinase that cleaves type IV collagen of the epithelial and vascular basement membrane and also stimulates release of VEGF from ECM-sequestered pools. Benign tumors of the breast, colon, and stomach show little type IV collagenase activity, whereas their malignant counterparts overexpress this enzyme. Concurrently, the levels of metalloproteinase inhibitors are reduced so that the balance is tilted greatly toward tissue degradation. Indeed, overexpression of MMPs and other proteases has been reported for many malignant tumors.


Changes in attachment of tumor cells to ECM proteins Normal epithelial cells have receptors, such as integrins, for basement membrane laminin and collagens that are polarized at their basal surface; these receptors help to maintain the cells in a resting, differentiated state. Loss of adhesion in normal cells leads to induction of apoptosis, while, not surprisingly, tumor cells are resistant to this form of cell death. Additionally, the matrix itself is modified in ways that promote invasion and metastasis. For example, cleavage of the basement membrane proteins, collagen IV, and laminin by MMP-2 or MMP-9 generates novel sites that bind to receptors on tumor cells and stimulate migration.

Locomotion is the final step of invasion, propelling tumor cells through the degraded basement membranes and zones of matrix proteolysis Migration is a complex, multistep process that involves many families of receptors and signaling proteins that eventually impinge on the actin cytoskeleton. Such movement seems to be potentiated and directed by tumor cell–derived cytokines, such as autocrine motility factors. In addition, cleavage products of matrix components (e. g. , collagen, laminin) and some growth factors (e. g. , insulin-like growth factors I and II) have chemotactic activity for tumor cells. Stromal cells also produce paracrine effectors of cell motility, such as hepatocyte growth factor/scatter factor (HGF/SCF), which binds to receptors on tumor cells. Concentrations of HGF/SCF are elevated at the advancing edges of the highly invasive brain tumor glioblastoma multiforme, supporting their role in motility.

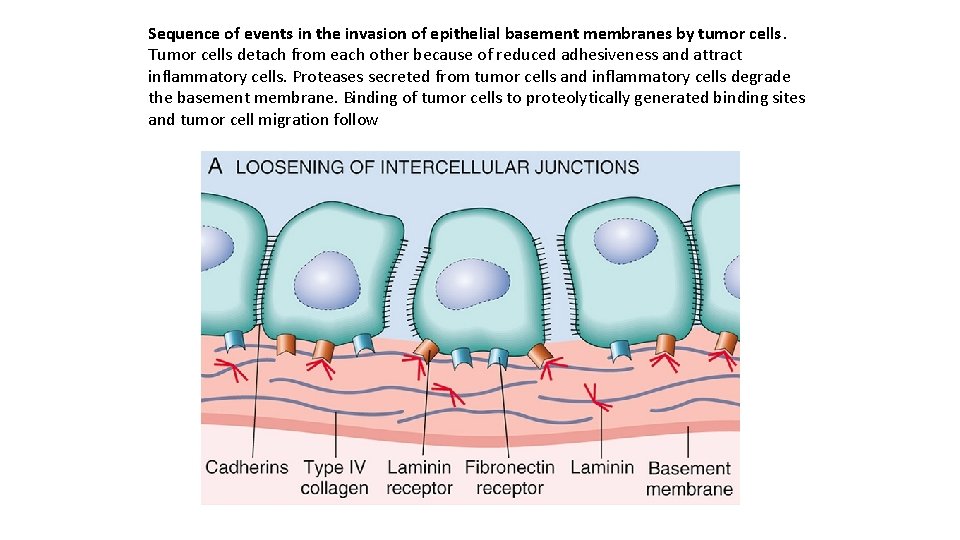
Sequence of events in the invasion of epithelial basement membranes by tumor cells. Tumor cells detach from each other because of reduced adhesiveness and attract inflammatory cells. Proteases secreted from tumor cells and inflammatory cells degrade the basement membrane. Binding of tumor cells to proteolytically generated binding sites and tumor cell migration follow



Vascular Dissemination and Homing of Tumor Cells Because of their invasive properties, tumor cells frequently escape their sites of origin and enter the circulation. It is now recognized from studies of “liquid biopsies, ” blood samples taken from patients with solid tumors, that millions of tumor cells are shed daily from even small cancers; hence both tumor cells and tumor-derived DNA can be detected in circulation. Most of these tumor cells circulate as single cells, while others form emboli by aggregating and adhering to circulating blood elements, particularly platelets. Given the ease with which tumor cells access the circulation, it is apparent that the ability of cancers cells to leave the circulation, invade, and grow to clinically significant sizes at other sites in the body is (fortunately for the patient) highly inefficient.

Several factors seem to limit the metastatic potential of circulating tumor cells While in the circulation, tumor cells are vulnerable to destruction by host immune cells and the process of adhesion to normal vascular beds and invasion of normal distant tissues may be much more difficult than the escape of tumor cells from the cancer. Even following extravasation, tumor cells that have been selected for growth in the originating tissue may find it difficult to grow in a second site due to lack of critical stromal support or because of recognition and suppression by resident immune cells. Indeed, the concept of tumor dormancy, referring to the prolonged survival of micrometastases without progression, is well described in melanoma and in breast and prostate carcinoma. Dormancy of tumor cells at distant sites may well be the last defense against clinically significant metastatic disease.

Despite these limiting factors, if neglected, virtually all malignant tumors will eventually produce macroscopic metastases. The site at which metastases appear is related to two factors: the anatomic location and vascular drainage of the primary tumor, and the tropism of particular tumors for specific tissues. Most metastases occur in the first capillary bed available to the tumor, hence the frequency of metastases to liver and lung. Many observations, however, suggest that natural pathways of drainage do not wholly explain the distribution of metastases. For example, prostatic carcinoma preferentially spreads to bone, bronchogenic carcinomas tend to involve the adrenal glands and the brain, and neuroblastomas spread to the liver and bones. Such organ tropism may be related to the following mechanisms:

• Tumor cells may have adhesion molecules whose ligands are expressed preferentially on the endothelial cells of the target organ. • Chemokines may have an important role in determining the target tissues for metastasis. For instance, many cancers express the chemokine receptor CXCR 4, which has been implicated in the extravasation of circulating tumor cells originating from tumors such as breast cancer. • In some cases, the target tissue may be a nonpermissive environment, “unfavorable soil, ” so to speak, for the growth of tumor seedlings. For example, although they are well vascularized, skeletal muscle and spleen are rarely sites of metastasis.


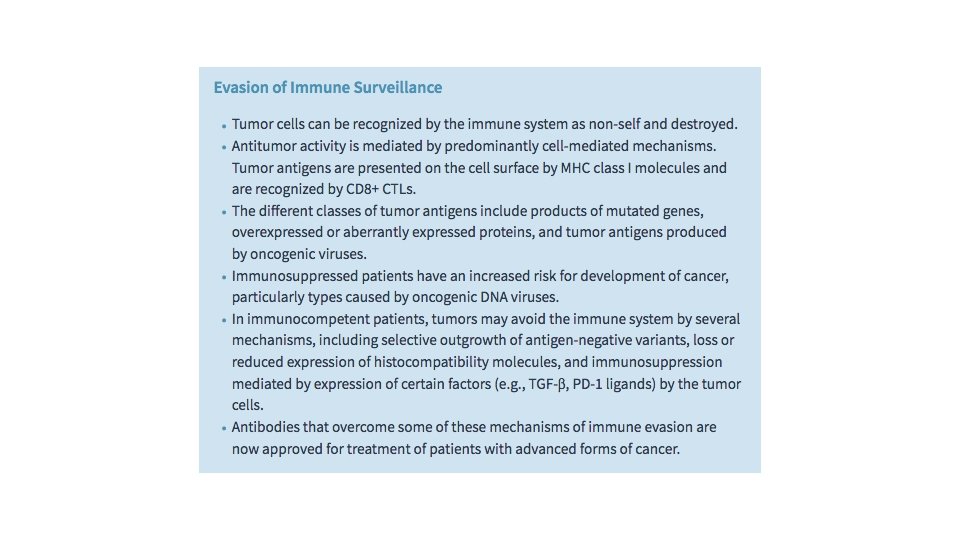
Evasion of Immune Surveillance Long one of the “holy grails” of oncology, the promise of therapies that enable the host immune system to recognize and destroy cancer cells is finally coming to fruition, largely due to a clearer understanding of the mechanisms by which cancer cells evade the host response. Paul Ehrlich first conceived the idea that tumor cells can be recognized as “foreign” and eliminated by the immune system. Subsequently, Lewis Thomas and Macfarlane Burnet formalized this concept by coining the term immune surveillance, based on the premise that a normal function of the immune system is to constantly “scan” the body for emerging malignant cells and destroy them. This idea has been supported by many observations—the direct demonstration of tumorspecific T cells and antibodies in patients; data showing that the extent and quality of immune infiltrates in cancers often correlates with outcome; the increased incidence of certain cancers in immunodeficient people and mice; and most recently and most directly, the dramatic success of immunotherapy in the treatment of several cancers.

The specific factors that govern the outcome of interactions between tumor cells and the host immune system are numerous and are still being defined. In the face of this complexity, it is helpful to consider a few overarching principles: Cancer cells express a variety of antigens that stimulate the host immune system, which appears to have an important role in preventing the emergence of cancers. • Despite the antigenicity of cancer cells, the immune response to established tumors is ineffective, and in some instances may actually promote cancer growth, due to acquired changes that allow cancer cells to evade anti-tumor responses and foster pro-tumor responses. • Defining mechanisms of immune evasion and “immunomanipulation” by cancer cells has led to effective new immunotherapies that work by reactivating latent host immune responses. •

Tumor Antigens In some instances, unmutated proteins expressed by tumor cells also can stimulate the host immune response. One such antigen is tyrosinase, an enzyme involved in melanin biosynthesis that is expressed only in normal melanocytes and melanomas. It may be surprising that the immune system is able to respond to this normal self-antigen. The probable explanation is that tyrosinase is normally produced in such small amounts and in so few normal cells that it is not recognized by the immune system and fails to induce tolerance. Another group of tumor antigens, the cancer-testis antigens, are encoded by genes that are silent in all adult tissues except germ cells in the testis—hence their name. Although the protein is present in the testis it is not expressed on the cell surface in a form that can be recognized by CD 8+ T cells, because sperm do not express MHC class I molecules. Thus, for all practical purposes these antigens are tumor specific and are therefore capable of stimulating anti-tumor immune responses.

An additional important class of tumor antigens consists of viral proteins that are expressed in cancer cells transformed by oncogenic viruses. The most potent of these antigens are proteins produced by cells that are latently infected with DNA viruses, the most important of which are human papilloma virus (HPV) and Epstein-Barr virus (EBV). There is abundant evidence that cytotoxic T lymphocytes (CTLs) recognize viral antigens and play important roles in surveillance against virus-induced tumors through their ability to recognize and kill virus-infected cells. Most notably, multiple cancers associated with oncogenic viruses, including HPV-associated cervical carcinoma and EBV-related B cell lymphomas, occur at significantly higher rates in individuals with defective T cell immunity, such as patients infected with HIV.

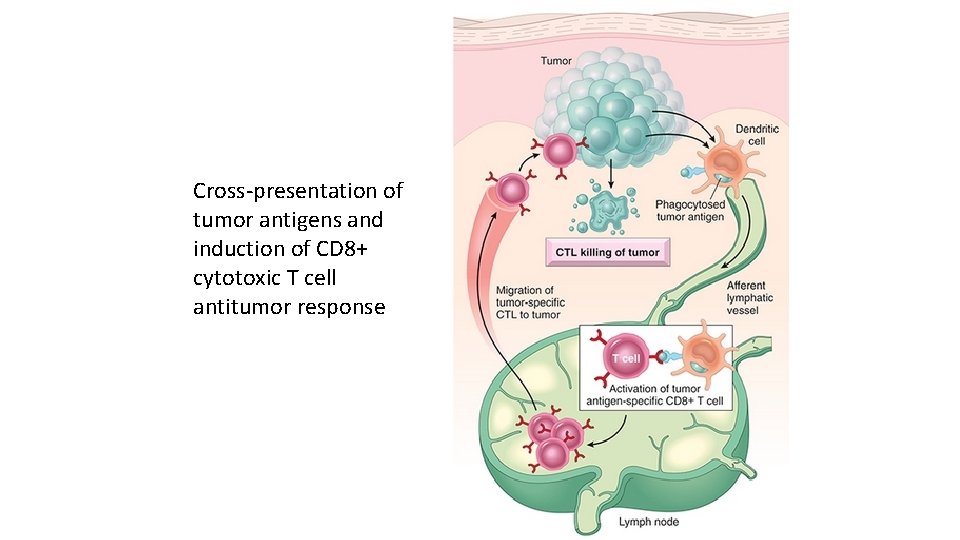
Cross-presentation of tumor antigens and induction of CD 8+ cytotoxic T cell antitumor response

Immune Evasion by Cancers Since the immune system is capable of recognizing and eliminating nascent cancers, it follows that tumors that reach clinically significant sizes must be composed of cells that are either invisible to the host immune system or that express factors that actively suppress host immunity. The term cancer immunoediting has been used to describe the ability of the immune system to promote the darwinian selection of the tumor subclones that are most able to avoid host immunity or even manipulate the immune system for their own malignant purposes. Since CTL responses appear to be the most important defense that the host has against tumors, it should come as no surprise that tumor cells show a variety of alterations that abrogate CTL responses. These include acquired mutations in β 2 -microglobulin that prevent the assembly of functional MHC class I molecules, and increased expression of a variety of proteins that inhibit CTL function. These proteins work by activating what is referred to as immune checkpoints, inhibitory pathways that normally are crucial for maintaining self-tolerance and controlling the size and duration of immune responses so as to minimize collateral tissue damage.

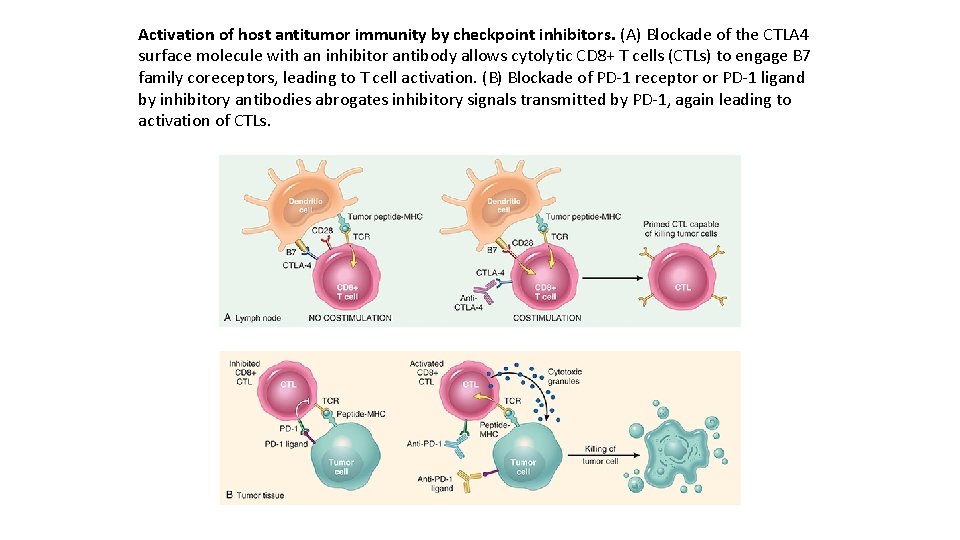
Activation of host antitumor immunity by checkpoint inhibitors. (A) Blockade of the CTLA 4 surface molecule with an inhibitor antibody allows cytolytic CD 8+ T cells (CTLs) to engage B 7 family coreceptors, leading to T cell activation. (B) Blockade of PD-1 receptor or PD-1 ligand by inhibitory antibodies abrogates inhibitory signals transmitted by PD-1, again leading to activation of CTLs.

The discovery of checkpoints that shut off anti-tumor immunity has led to the development of antibodies that block these checkpoints and release the brakes on the immune response. Current checkpoint blockade therapies have resulted in response rates of 10– 30% in a variety of solid tumors (melanoma, lung cancer, bladder cancer, and others), and even higher rates in some hematologic malignancies such as Hodgkin lymphoma. Because these checkpoints evolved to prevent responses to self antigens, it is not surprising that patients treated with checkpoint blockade therapy develop various autoimmune manifestations, such as colitis and other types of systemic inflammation. Most of these reactions can be controlled with antiinflammatory agents.


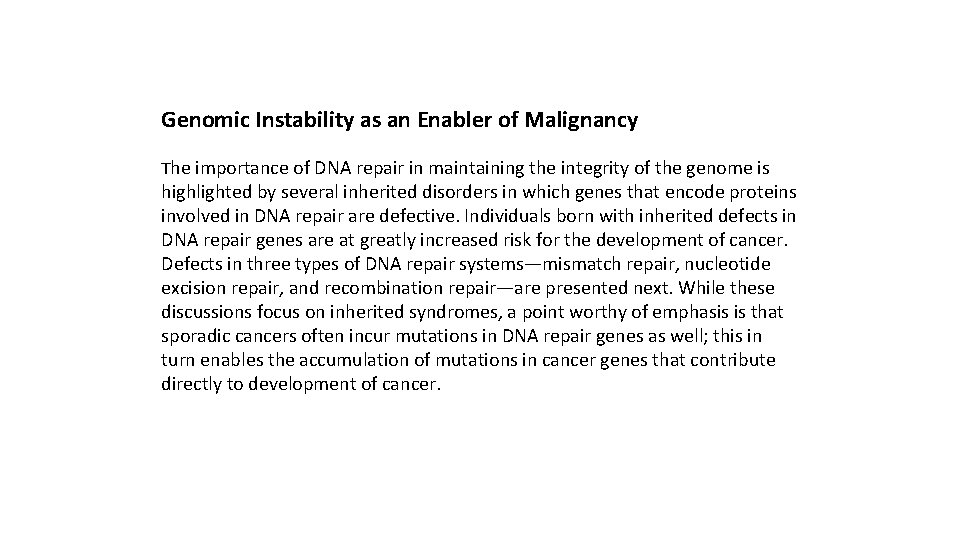
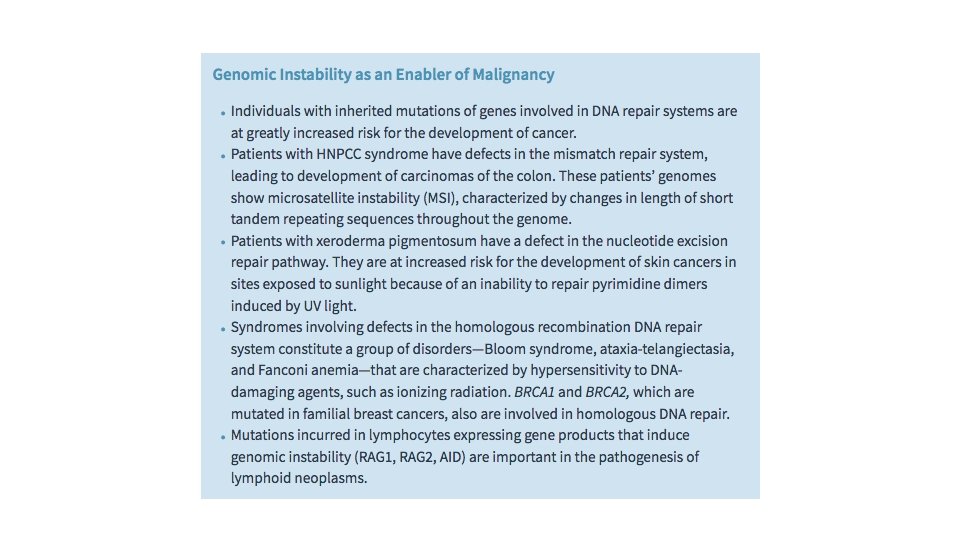
Genomic Instability as an Enabler of Malignancy The importance of DNA repair in maintaining the integrity of the genome is highlighted by several inherited disorders in which genes that encode proteins involved in DNA repair are defective. Individuals born with inherited defects in DNA repair genes are at greatly increased risk for the development of cancer. Defects in three types of DNA repair systems—mismatch repair, nucleotide excision repair, and recombination repair—are presented next. While these discussions focus on inherited syndromes, a point worthy of emphasis is that sporadic cancers often incur mutations in DNA repair genes as well; this in turn enables the accumulation of mutations in cancer genes that contribute directly to development of cancer.

Hereditary Nonpolyposis Colon Cancer Syndrome Hereditary nonpolyposis colon carcinoma (HNPCC) syndrome dramatically illustrates the role of DNA repair genes in predisposition to cancer. This disorder, characterized by familial carcinomas of the colon affecting predominantly the cecum and proximal colon, results from defects in genes involved in DNA mismatch repair. When a strand of DNA is being repaired, the products of mismatch repair genes act as “spell checkers. ” For example, if there is an erroneous pairing of G with T, rather than the normal A with T, the mismatch repair genes correct the defect. Without these “proofreaders, ” errors accumulate at an increased rate. Mutations in at least four mismatch repair genes have been found to underlie HNPCC. Each affected individual inherits one defective copy of one of several DNA mismatch repair genes and acquires the second “hit” in colonic epithelial cells. Thus, DNA repair genes affect cell growth only indirectly—by allowing mutations in other genes during the process of normal cell division. A characteristic finding in the genome of patients with mismatch repair defects is microsatellite instability (MSI). In normal people, the length of these microsatellites remains constant. By contrast, in patients with HNPCC, these satellites are unstable and increase or decrease in length. Although HNPCC accounts for only 2% to 4% of all colonic cancers, MSI can be detected in about 15% of sporadic cancers due to acquired mutations that disrupt the function of mismatch repair genes.


Xeroderma Pigmentosum Xeroderma pigmentosum is an autosomal recessive disorder caused by a defect in DNA repair that is associated with a greatly increased risk for cancers arising in sun-exposed skin. Ultraviolet (UV) rays in sunlight cause cross-linking of pyrimidine residues, preventing normal DNA replication. Such DNA damage is repaired by the nucleotide excision repair system. Several proteins are involved in nucleotide excision repair, and the inherited loss of any one of these can give rise to xeroderma pigmentosum.

Diseases with Defects in DNA Repair by Homologous Recombination A group of autosomal recessive disorders comprising Bloom syndrome, ataxiatelangiectasia, and Fanconi anemia is characterized by hypersensitivity to DNAdamaging agents, such as ionizing radiation (in Bloom syndrome and ataxiatelangiectasia), or to DNA cross-linking agents, such as nitrogen mustard (in Fanconi anemia). Their phenotype is complex and includes, in addition to predisposition to cancer, features such as neural symptoms (in ataxia-telangiectasia), anemia (in Fanconi anemia), and developmental defects (in Bloom syndrome). The gene mutated in ataxiatelangiectasia is ATM, which encodes a protein kinase that is important in “sensing” DNA damage caused by ionizing radiation and then directing p 53 to initiate the DNA damage response, as described earlier. Evidence for the role of DNA repair genes in the origin of cancer also comes from the study of hereditary breast cancer. Germ line mutations in two genes, BRCA 1 and BRCA 2, account for 50% of cases of familial breast cancer. In addition to breast cancer, women with BRCA 1 mutations have a substantially higher risk for developing epithelial ovarian cancers, and men have a slightly higher risk for developing prostate cancer.



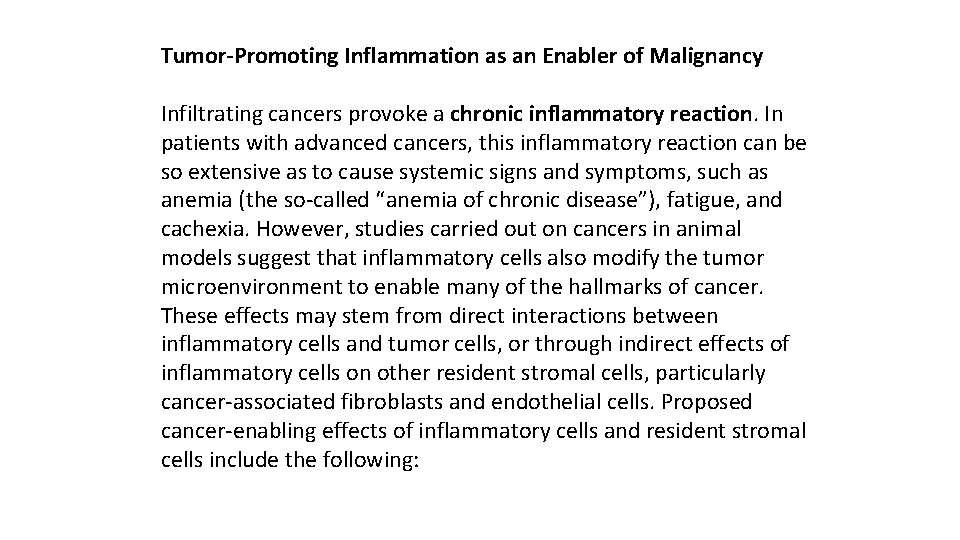
Tumor-Promoting Inflammation as an Enabler of Malignancy Infiltrating cancers provoke a chronic inflammatory reaction. In patients with advanced cancers, this inflammatory reaction can be so extensive as to cause systemic signs and symptoms, such as anemia (the so-called “anemia of chronic disease”), fatigue, and cachexia. However, studies carried out on cancers in animal models suggest that inflammatory cells also modify the tumor microenvironment to enable many of the hallmarks of cancer. These effects may stem from direct interactions between inflammatory cells and tumor cells, or through indirect effects of inflammatory cells on other resident stromal cells, particularly cancer-associated fibroblasts and endothelial cells. Proposed cancer-enabling effects of inflammatory cells and resident stromal cells include the following:

Release of factors that promote proliferation. Infiltrating leukocytes and activated stromal cells have been shown to secrete a wide variety of growth factors, such as EGF, and proteases that can liberate growth factors from the extracellular matrix (ECM). Removal of growth suppressors. As mentioned earlier, the growth of epithelial cells is suppressed by cell–cell and cell– ECM interactions. Proteases released by inflammatory cells can degrade the adhesion molecules that mediate these interactions, removing a barrier to growth.

Enhanced resistance to cell death. Detachment of epithelial cells from basement membranes and from cell–cell interactions can lead to a particular form of programmed cell death called anoikis. It is suspected that tumor-associated macrophages may prevent anoikis by expressing adhesion molecules such as integrins that promote direct physical interactions with tumor cells. Angiogenesis. Inflammatory cells release numerous factors, including VEGF, that stimulate angiogenesis. Invasion and metastasis. Proteases released from macrophages foster tissue invasion by remodeling the ECM, while factors such as TNF and EGF may directly stimulate tumor cell motility. As mentioned earlier, other factors released from stromal cells such as TGF-β may promote epithelial-mesenchymal transition (EMT), which may be a key event in the process of invasion and metastasis.

Evasion of immune destruction. A variety of soluble factors released by macrophages and other stromal cells are believed to contribute to an immunosuppressive tumor microenvironment. The leading suspects include TGF-β and other factors that either favor the recruitment of immunosuppressive T regulatory cells or suppress the function of CD 8+ cytotoxic T cells. Furthermore, there is abundant evidence in cancer models and emerging evidence in human disease that advanced cancers contain mainly alternatively activated (M 2) macrophages. M 2 macrophages produce cytokines that promote angiogenesis, fibroblast proliferation, and collagen deposition, all of which are commonly observed in invasive cancers and in healing wounds, giving rise to the notion that cancers are like “wounds that do not heal. ”

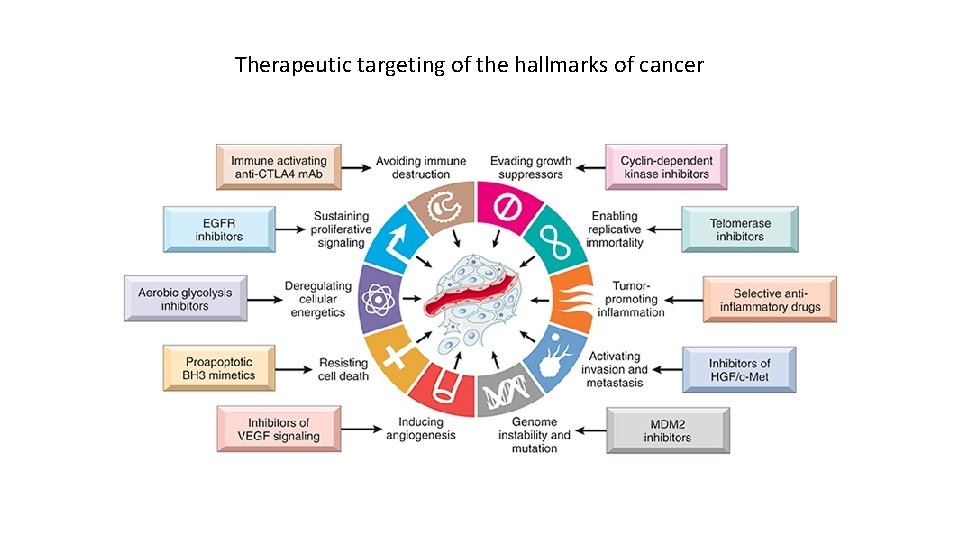
Therapeutic targeting of the hallmarks of cancer

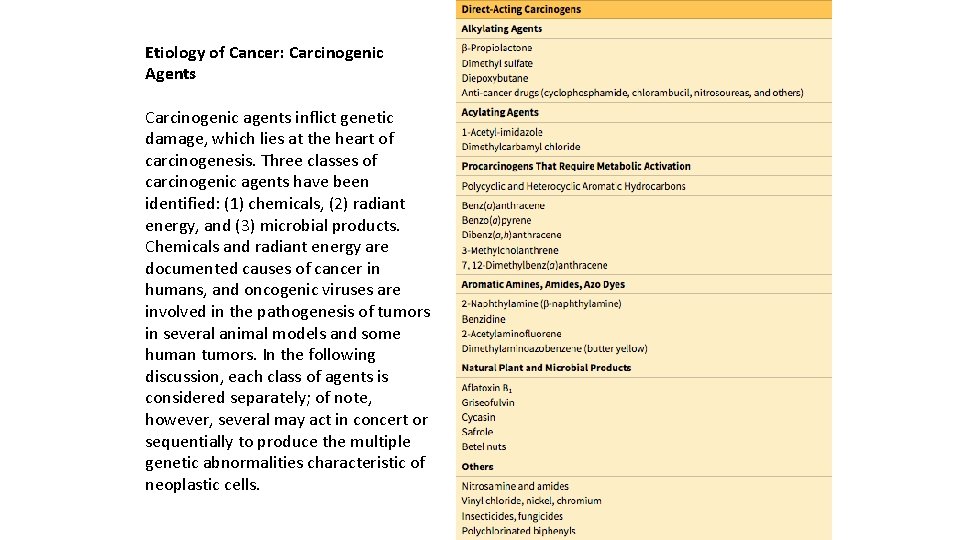
Etiology of Cancer: Carcinogenic Agents Carcinogenic agents inflict genetic damage, which lies at the heart of carcinogenesis. Three classes of carcinogenic agents have been identified: (1) chemicals, (2) radiant energy, and (3) microbial products. Chemicals and radiant energy are documented causes of cancer in humans, and oncogenic viruses are involved in the pathogenesis of tumors in several animal models and some human tumors. In the following discussion, each class of agents is considered separately; of note, however, several may act in concert or sequentially to produce the multiple genetic abnormalities characteristic of neoplastic cells.

Direct-Acting Agents Direct-acting agents require no metabolic conversion to become carcinogenic. They are typically weak carcinogens but are important because some of them are cancer chemotherapy drugs (e. g. , alkylating agents) used in regimens that may cure certain types of cancer (e. g. , Hodgkin lymphoma), only to evoke a subsequent, second form of cancer, usually leukemia. This situation is even more tragic when the initial use of such agents has been for non-neoplastic disorders, such as rheumatoid arthritis or granulomatosis with polyangiitis. The associated risk for induced cancer is low, but its existence dictates judicious use of such agents. Indirect-Acting Agents The designation indirect-acting refers to chemicals that require metabolic conversion to an ultimate carcinogen. Some of the most potent indirect chemical carcinogens are polycyclic hydrocarbons that are created with burning of fossil fuels, plant, and animal material. For example, benzo[a]pyrene and other carcinogens formed during the combustion of tobacco are implicated in the causation of lung cancer, and in olden days, benzo[a]pyrene created during the burning of coal was likely responsible for the high incidence of scrotal cancer in chimney sweeps. Polycyclic hydrocarbons also may be produced from animal fats during the process of broiling meats and are present in smoked meats and fish. In the body, benzo[a]pyrene is metabolized to epoxides, which form covalent adducts (addition products) with molecules in the cell, principally DNA, but also with RNA and proteins.

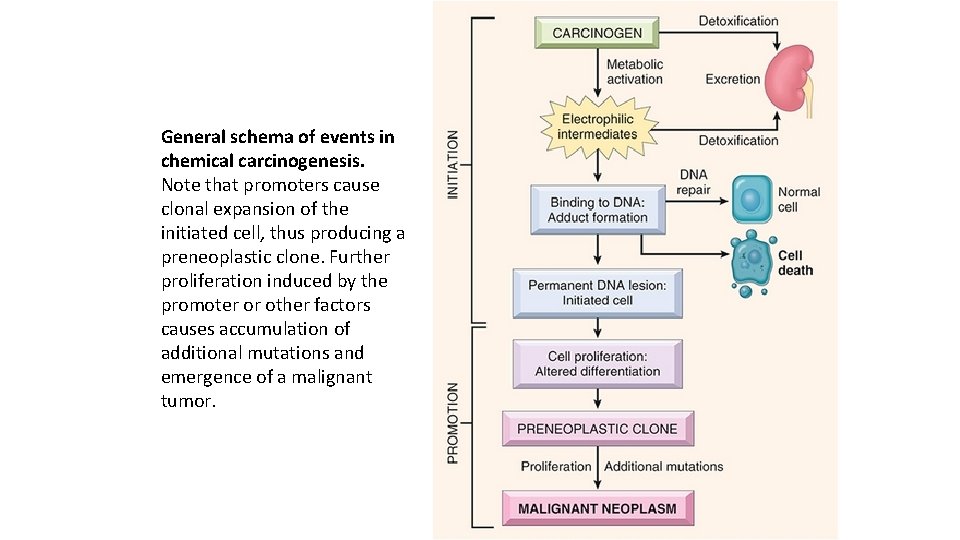
General schema of events in chemical carcinogenesis. Note that promoters cause clonal expansion of the initiated cell, thus producing a preneoplastic clone. Further proliferation induced by the promoter or other factors causes accumulation of additional mutations and emergence of a malignant tumor.


Oncogenic RNA Viruses Although the study of animal retroviruses has provided spectacular insights into the molecular basis of cancer, only one human retrovirus, human T-cell leukemia virus type 1 (HTLV-1), is firmly implicated in the pathogenesis of cancer in humans. HTLV-1 causes adult T-cell leukemia/lymphoma (ATLL), a tumor that is endemic in certain parts of Japan, the Caribbean basin, South America, and Africa, and found sporadically elsewhere, including the United States


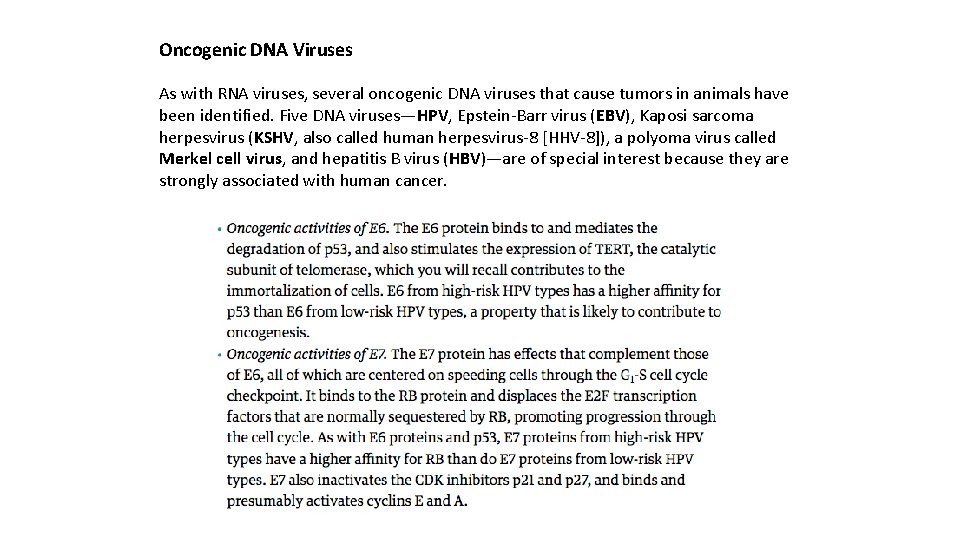
Oncogenic DNA Viruses As with RNA viruses, several oncogenic DNA viruses that cause tumors in animals have been identified. Five DNA viruses—HPV, Epstein-Barr virus (EBV), Kaposi sarcoma herpesvirus (KSHV, also called human herpesvirus-8 [HHV-8]), a polyoma virus called Merkel cell virus, and hepatitis B virus (HBV)—are of special interest because they are strongly associated with human cancer.

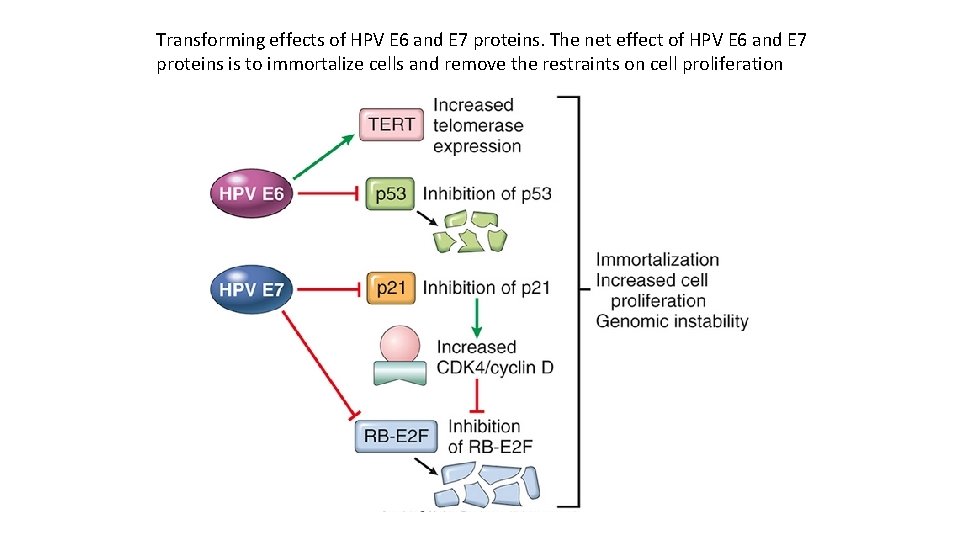
Transforming effects of HPV E 6 and E 7 proteins. The net effect of HPV E 6 and E 7 proteins is to immortalize cells and remove the restraints on cell proliferation


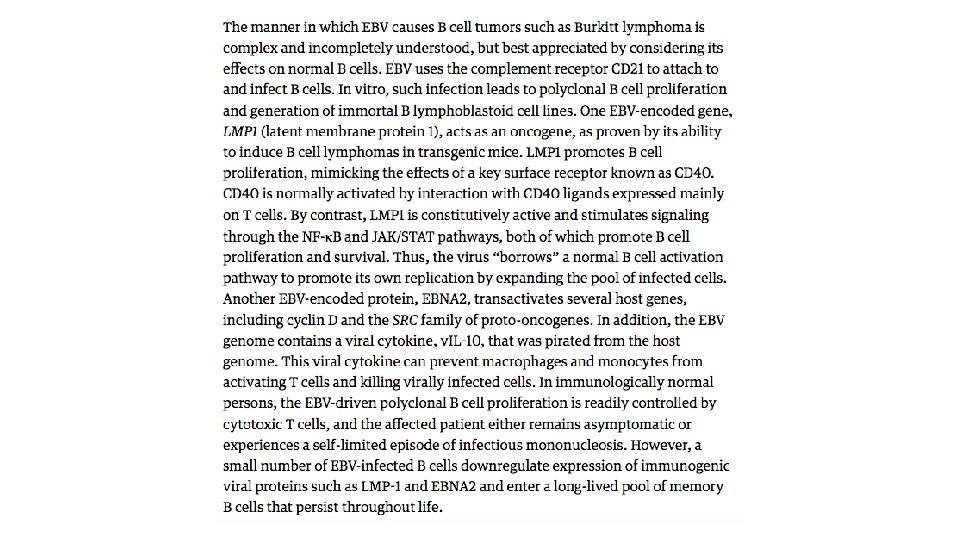
Epstein-Barr Virus EBV, a member of the herpesvirus family, was the first virus linked to a human tumor, Burkitt lymphoma is an aggressive tumor that is endemic in certain parts of Africa and occurs sporadically elsewhere. In endemic areas, the tumor cells in virtually all affected patients carry the EBV genome. Since its initial discovery in Burkitt lymphoma some 50 years ago, EBV has been detected within the cells of a surprisingly diverse list of other tumors, including most nasopharyngeal carcinomas and a subset of T cell lymphomas, NK cell lymphomas, gastric carcinomas, and even, in rare instances, sarcomas, mainly in the immunosuppressed.



Possible evolution of EBVinduced Burkitt lymphoma




Hepatitis B and Hepatitis C Viruses The epidemiologic evidence linking chronic HBV and hepatitis C virus (HCV) infection with hepatocellular carcinoma is strong. It is estimated that 70% to 85% of hepatocellular carcinomas worldwide are caused by HBV or HCV. However, the mode of action of these viruses in tumorigenesis is not fully elucidated. The HBV and HCV genomes do not encode any viral oncoproteins, and although the HBV DNA is integrated within the human genome, there is no consistent pattern of integration in liver cells. Indeed, the oncogenic effects of HBV and HCV are multifactorial, but the dominant effect seems to be immunologically mediated chronic inflammation with hepatocyte death, leading to regeneration and genomic damage. Although the immune system generally is thought to be protective, recent work has demonstrated that in the setting of unresolved chronic inflammation, as occurs in viral hepatitis or chronic gastritis caused by H. pylori, the immune response may become maladaptive, promoting tumorigenesis.









Grading and Staging of Cancer Methods to quantify the probable clinical aggressiveness of a given neoplasm and its apparent extent and spread in the individual patient are necessary for arriving at an accurate prognosis and for comparing end results of various treatment protocols. For instance, the results of treating well-differentiated thyroid adenocarcinomas localized to the thyroid gland will on average be very different from those obtained from treating highly anaplastic thyroid cancers that have invaded the neck organs. Systems have been developed to express, at least in semiquantitative terms, the level of differentiation, or grade, and extent of spread of a cancer within the patient, or stage, as parameters of the clinical gravity of the disease. Of note, when compared with grading, staging has proved to be of greater clinical value.



Laboratory Diagnosis Fine-needle aspiration of tumors is another approach that is widely used. It involves aspiration of cells from a mass, followed by cytologic examination of the cells after they have been spread out on a slide. This procedure is used most commonly with readily palpable lesions affecting the breast, thyroid gland, lymph nodes, and salivary glands. Modern imaging techniques permit extension of the method to deeper structures, such as the liver, pancreas, and pelvic lymph nodes. Use of this diagnostic modality obviates surgery and its attendant risks. Although it entails some difficulties, such as small sample size and sampling errors, in experienced hands it can be reliable, rapid, and useful.


Cytologic (Papanicolaou) smears provide another method for the detection of cancer. Historically, this approach has been used widely for discovery of carcinoma of the cervix, often at an in situ stage, but now it is used to investigate many other forms of suspected malignancy, such as endometrial carcinoma, bronchogenic carcinoma, bladder and prostate tumors, and gastric carcinomas; for the identification of tumor cells in abdominal, pleural, joint, and cerebrospinal fluids; and, less commonly, for evaluation of other forms of neoplasia. Neoplastic cells are less cohesive than others and are therefore shed into fluids or secretions. The shed cells are evaluated for features of anaplasia indicative of their origin from a tumor. The gratifying control of cervical cancer is the best testament to the value of the cytologic method.

(A) Normal Papanicolaou smear from the uterine cervix. Large, flat cells with small nuclei are typical.

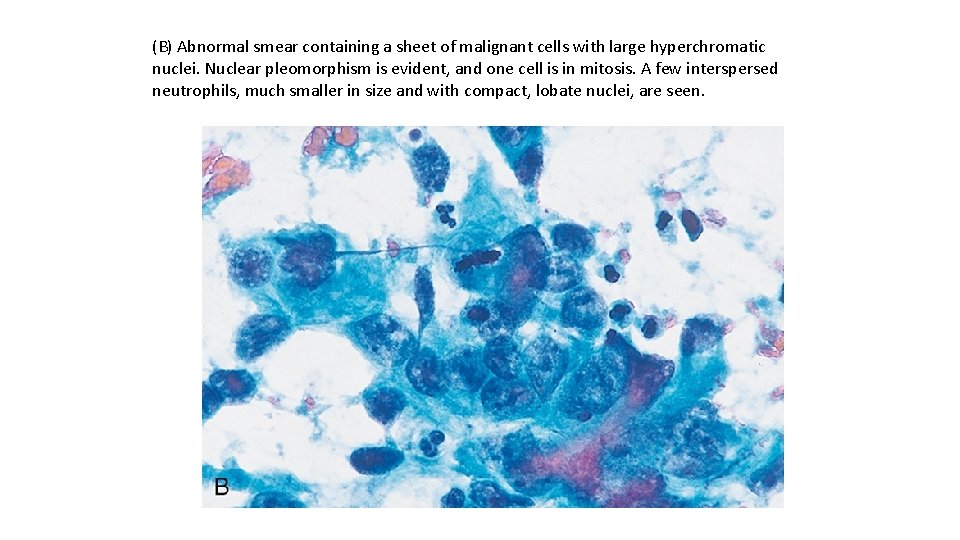
(B) Abnormal smear containing a sheet of malignant cells with large hyperchromatic nuclei. Nuclear pleomorphism is evident, and one cell is in mitosis. A few interspersed neutrophils, much smaller in size and with compact, lobate nuclei, are seen.

Tumor Markers Biochemical assays for tumor-associated enzymes, hormones, and other tumor markers in the blood cannot be utilized for definitive diagnosis of cancer; however, they are used with varying success as screening tests and have utility in monitoring the response to therapy or detecting disease recurrence. PSA, used to screen for prostatic adenocarcinoma, is one of the most frequently used tumor markers in clinical practice. Prostatic carcinoma can be suspected when elevated levels of PSA are found in the blood. However, PSA screening also highlights problems encountered with use of virtually every tumor marker. Although PSA levels often are elevated in cancer, PSA levels also may be elevated in benign prostatic hyperplasia. Furthermore, there is no PSA level that ensures that a patient does not have prostate cancer. Thus, the PSA test suffers from both low sensitivity and low specificity, and its use as a screening tool has become quite controversial.

The PSA assay is extremely valuable, however, for detecting residual disease or recurrence following treatment for prostate cancer. Other tumor markers used in clinical practice include carcinoembryonic antigen (CEA), which is elaborated by carcinomas of the colon, pancreas, stomach, and breast, and alpha fetoprotein (AFP), which is produced by hepatocellular carcinomas, yolk sac remnants in the gonads, and occasionally teratocarcinomas and embryonal cell carcinomas. Like PSA, CEA and AFP can be elevated in a variety of non-neoplastic conditions and thus also lack the specificity and sensitivity required for the early detection of cancers, but they may be useful in monitoring disease once the diagnosis is established. With successful resection of the tumor, these markers disappear from the serum; their reappearance almost always signifies recurrence.


Molecular Diagnosis An increasing number of molecular techniques are being used for the diagnosis of tumors and for predicting their behavior. Diagnosis of malignancy. Because each T cell and B cell has unique antigen receptor gene rearrangements, polymerase chain reaction (PCR)–based detection of rearranged T-cell receptor or immunoglobulin genes allows monoclonal (neoplastic) and polyclonal (reactive) proliferations to be distinguished. Many hematopoietic neoplasms, as well as a few solid tumors, are defined by particular translocations, so the diagnosis can be made by detection of such translocations. For example, fluorescence in situ hybridization (FISH) or PCR analysis can be used to detect translocations characteristic of Ewing sarcoma and several leukemias and lymphomas. PCR-based detection of BCR-ABL transcripts can confirm the diagnosis of chronic myeloid leukemia. Finally, certain hematologic malignancies are now defined by the presence of point mutations in particular oncogenes. For example, as mentioned earlier, the diagnosis of another myeloid neoplasm called polycythemia vera requires the identification of specific mutations in JAK 2, a gene that encodes a nonreceptor tyrosine kinase.


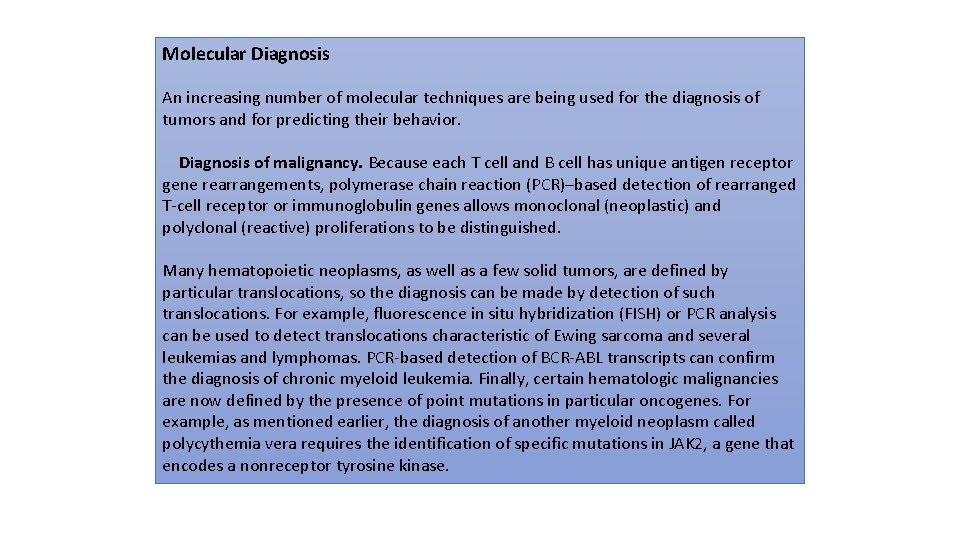
Therapeutic decision-making. Therapies that directly target specific mutations are increasingly being developed, and thus detection of such mutations in a tumor can guide the development of targeted therapy, as discussed later. It is now becoming evident that certain targetable mutations transgress morphologic categories. One example involves a valine for glutamate substitution in amino acid 600 (V 600 E) of the serine/threonine kinase BRAF, which you will recall lies downstream of RAS in the growth factor signaling pathway. Melanomas with the V 600 E BRAF mutation respond well to BRAF inhibitors, whereas melanomas without this mutation show no response. Subsequently, it was realized that the same V 600 E mutation is also present in a subset of many other diverse cancers, including carcinomas of the colon and thyroid gland, most hairy cell leukemias, and many cases of Langerhans cell histiocytosis. These tumors are morphologically diverse and have distinct cells of origin, but they share identical oncogenic lesions in a common pro-growth pathway.

Diverse tumor types that share a common mutation, BRAF (V 600 E), may be candidates for treatment with the same BRAF inhibitors

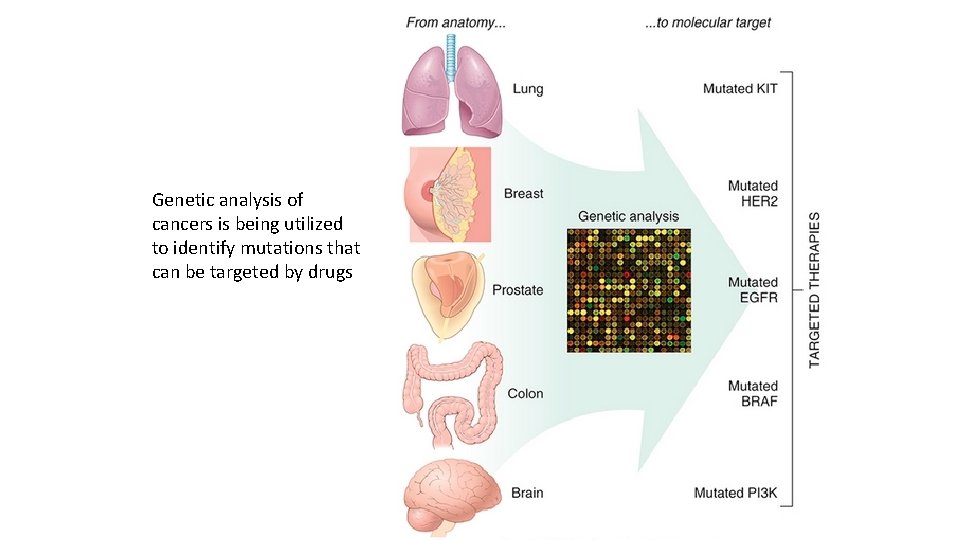
Genetic analysis of cancers is being utilized to identify mutations that can be targeted by drugs
 Cancer metastasis
Cancer metastasis Metastasis hepaticas multiples
Metastasis hepaticas multiples Metastasis process
Metastasis process Metastasis process
Metastasis process Apoptosis
Apoptosis Xleqxisfp definition
Xleqxisfp definition Roman invasion of carthage
Roman invasion of carthage Bay of pigs invasion on map
Bay of pigs invasion on map Culture invasion
Culture invasion Hyksos invasion of egypt
Hyksos invasion of egypt Segment invasion plan
Segment invasion plan Benign and malignant tumors
Benign and malignant tumors Example of invasion games
Example of invasion games Boundary invasion
Boundary invasion Contoh olahraga invasi
Contoh olahraga invasi What does this mean
What does this mean Invasion from mars characters
Invasion from mars characters The americas on the eve of invasion
The americas on the eve of invasion Which landforms protected the cities from invasions?
Which landforms protected the cities from invasions? Consecuencias de la invasión norteamericana de 1898
Consecuencias de la invasión norteamericana de 1898 How did the hun invasion weaken the roman empire?
How did the hun invasion weaken the roman empire? Invasion nocturna
Invasion nocturna Cuticle invasion
Cuticle invasion Steeven
Steeven Local invasion
Local invasion 1111 invasion des huns
1111 invasion des huns Local invasion
Local invasion Ptca
Ptca Bowland maths alien invasion
Bowland maths alien invasion Plebeians
Plebeians Inchon invasion map
Inchon invasion map Led zeppelin british invasion
Led zeppelin british invasion Design invasion
Design invasion Inchon invasion map
Inchon invasion map Invasion of kuwait
Invasion of kuwait Ruhr invasion
Ruhr invasion Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Major endocrine glands male and female
Major endocrine glands male and female Chapter 16 matching questions 6-10
Chapter 16 matching questions 6-10