Introductory Chemistry 2 Unit 2 Chapter 2 Chemical








- Slides: 43

Introductory Chemistry 2 Unit 2 Chapter 2

Chemical Elements • • Fundamental unit in chemistry Cannot be broken down by chemical means 112 elements total Use 1 -2 letter symbols for each e. g. C= carbon, Na = sodium, Cl = chorine. • • • 26 normally present in your body 4 major ones & 8 others significant (see table 2. 1) Unit 2 • • 2

Atoms • • 2 Lowest unit of an element Nucleus-protons (+), neutrons (0) Surrounded by Electrons (-) Total charge is neutral- • • Proton number=atomic numberdefines element Unit 2 Protons # = electron #

• • Atoms interact in characteristic ways Describing this is chemistry When two or more atoms are held together with chemical bonds the result is a molecule. Described by the molecular formula 2 Unit 2 Ions, Molecules & Compounds

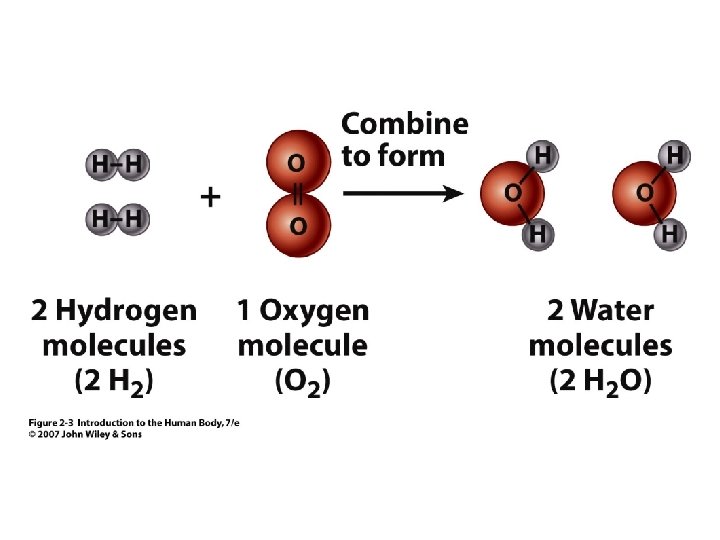
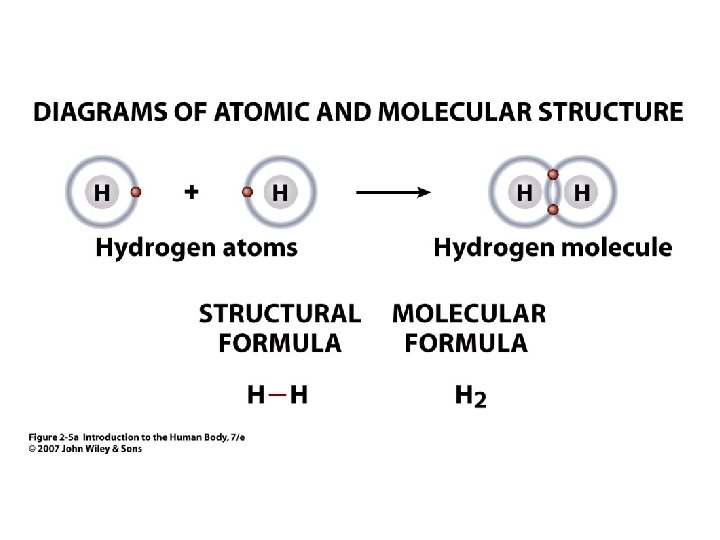
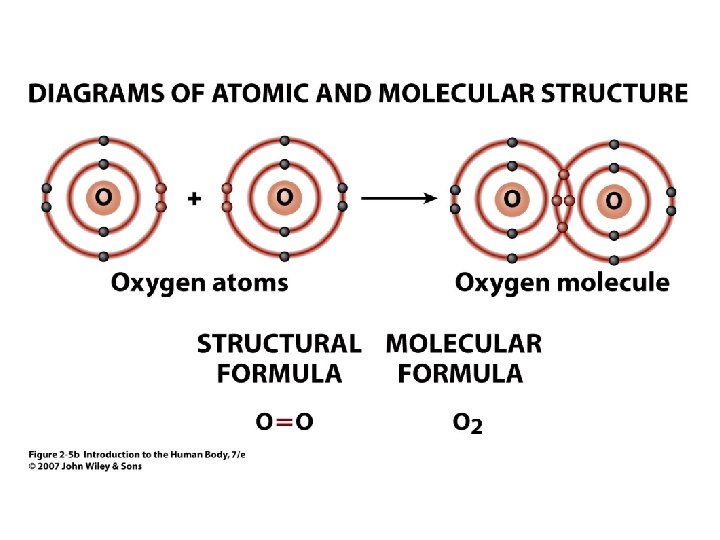
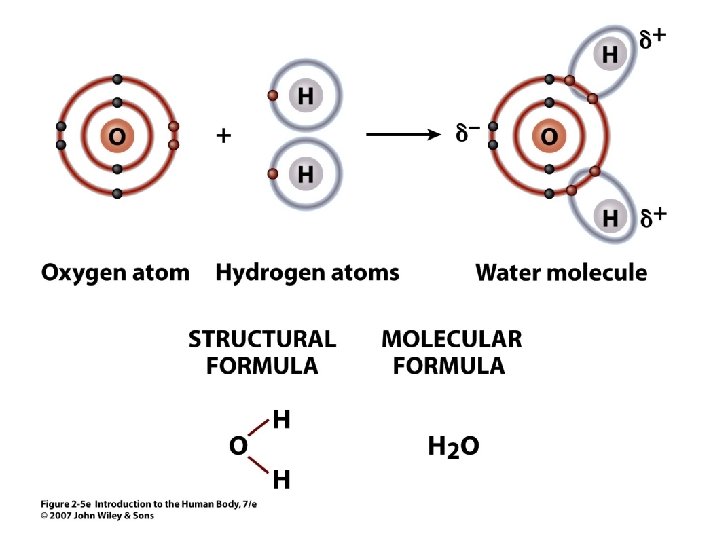
Molecular Formula • 2 Example: O 2 = oxygen the gas molecule has 2 atoms of oxygen bound together • H 2 O = water • • Subscript = # of atoms of element Connected letters & numbers = molecule Unit 2 Molecule has 2 atoms of H (hydrogen) and 1 atom of O (oxygen)

Figure 2. 3

• • attraction between atoms to form attachments = molecules Electrons grouped into shells preferred number in outer shell leads to chemical activity Can be covalent, ionic, polar covalent and Hydrogen bonds 2 Unit 2 Bonding

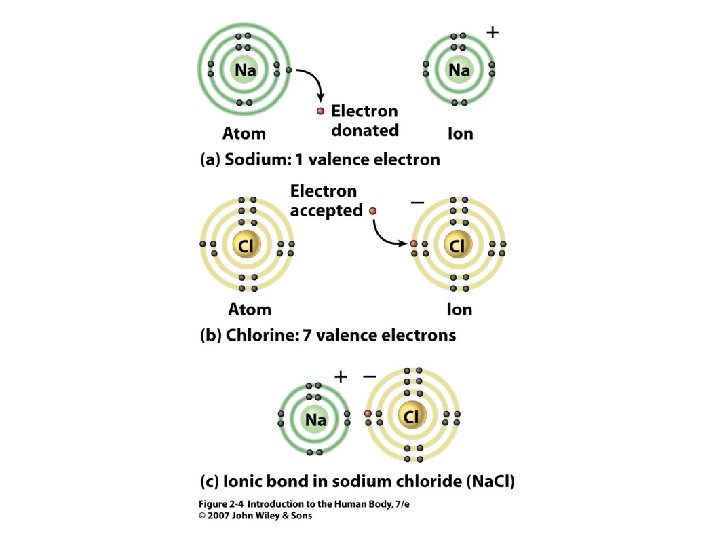
Ionic Bonds • 2 Can donate or accept electrons from another atom -> • • Opposite charges attract => bonding Ionic bonding Unit 2 Ions = atoms with a charge

Figure 2. 4

Covalent Bonds • 2 Can share electrons in outer shell > covalent bonds e. g. water, many organic compounds unequal sharing -> polar bond some partial charges on the molecule Unit 2 •

Figure 2. 5 a

Figure 2. 5 b

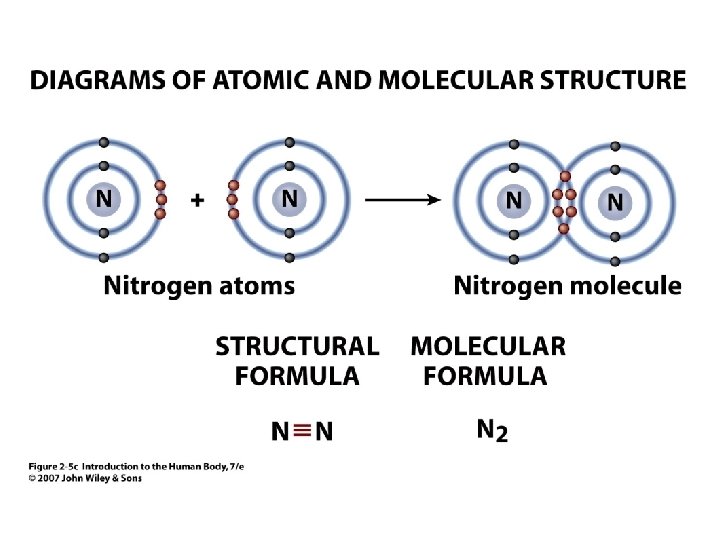
Figure 2. 5 c

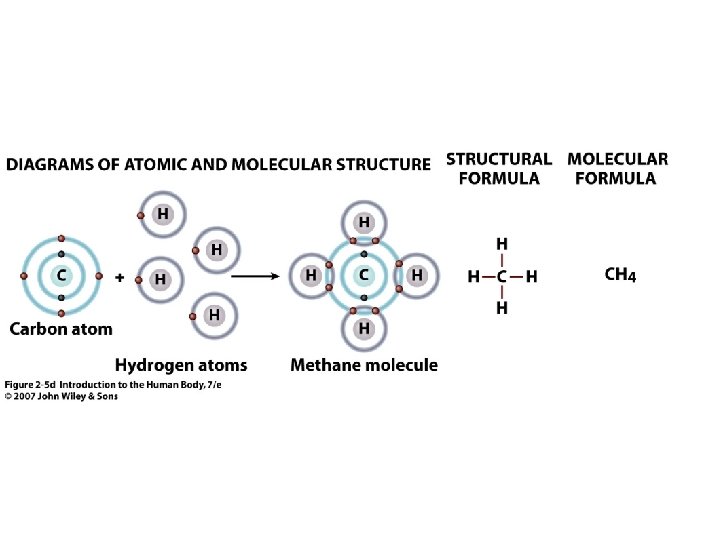
Figure 2. 5 d

Figure 2. 5 e

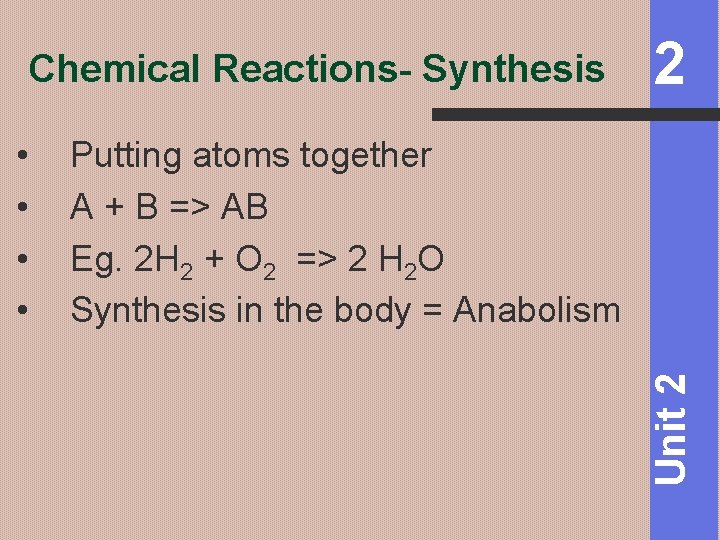
Chemical Reactions- Synthesis Putting atoms together A + B => AB Eg. 2 H 2 + O 2 => 2 H 2 O Synthesis in the body = Anabolism Unit 2 • • 2

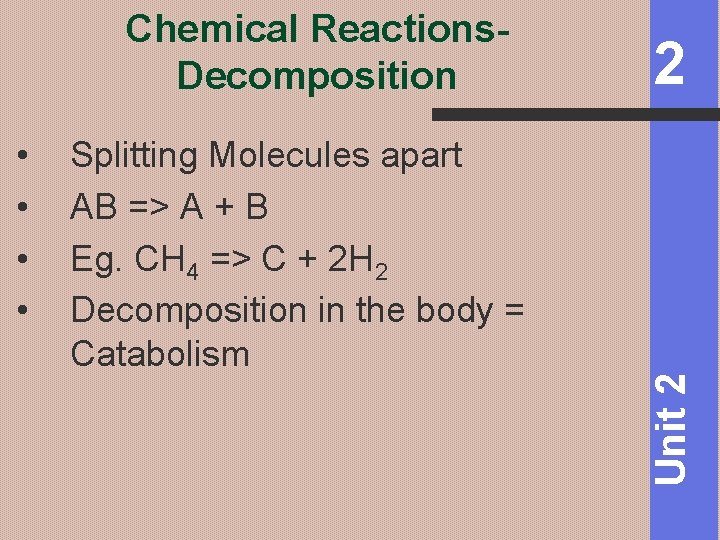
Chemical Reactions. Decomposition Splitting Molecules apart AB => A + B Eg. CH 4 => C + 2 H 2 Decomposition in the body = Catabolism Unit 2 • • 2

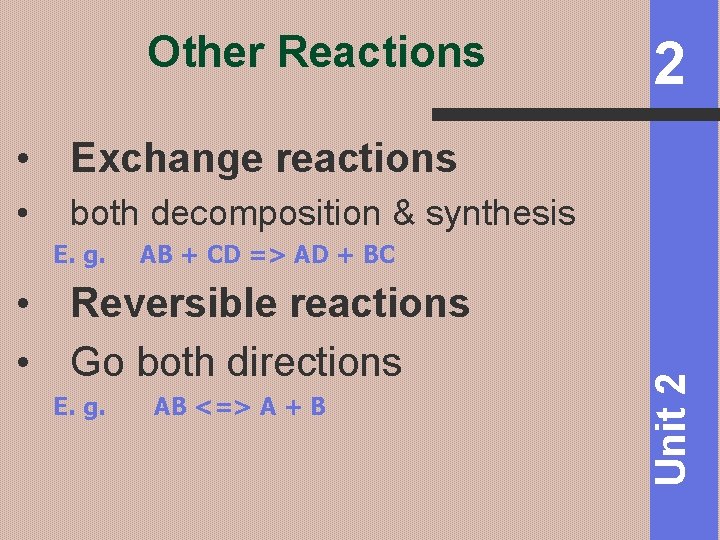
Other Reactions 2 • Exchange reactions both decomposition & synthesis E. g. AB + CD => AD + BC • Reversible reactions • Go both directions E. g. AB <=> A + B Unit 2 •

• • • Good solvent for some molecules Dissolve = Hydrophilic molecules Don’t dissolve = Hydrophobic molecules Participates in chemical reactions Absorbs & releases heat slowly Needs large amount of heat to evaporate 2 Unit 2 Nature of Water

• • • Acid dissolves => H+ (1 or more) Base dissolves => OH- ( 1 or more) Acid plus base react => salt • E. g. HCL + KOH => KCL + H 2 O acid base salt 2 Unit 2 Acid, Base & Salts

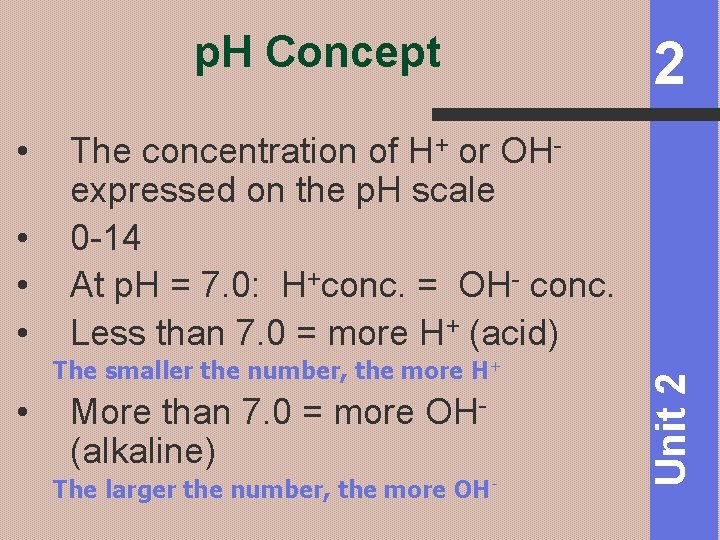
p. H Concept • • The concentration of H+ or OHexpressed on the p. H scale 0 -14 At p. H = 7. 0: H+conc. = OH- conc. Less than 7. 0 = more H+ (acid) The smaller the number, the more H+ More than 7. 0 = more OH- (alkaline) The larger the number, the more OH- Unit 2 • 2

Organic Compounds Carbohydrates Lipids Proteins Nucleic acids Unit 2 • • 2

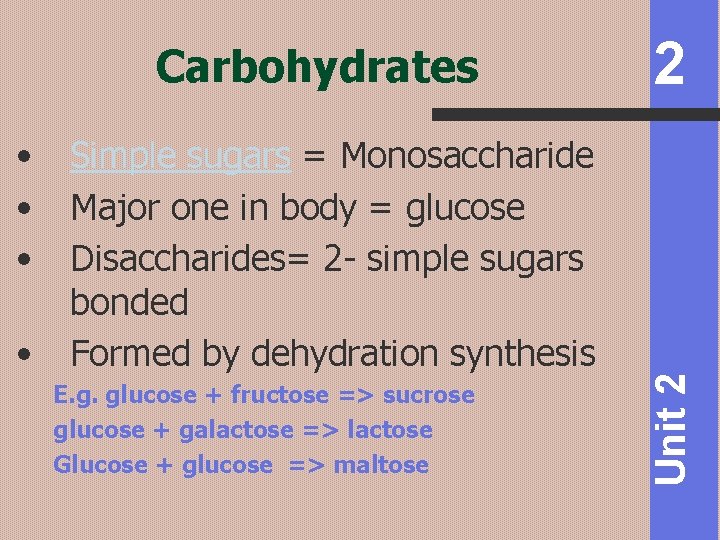
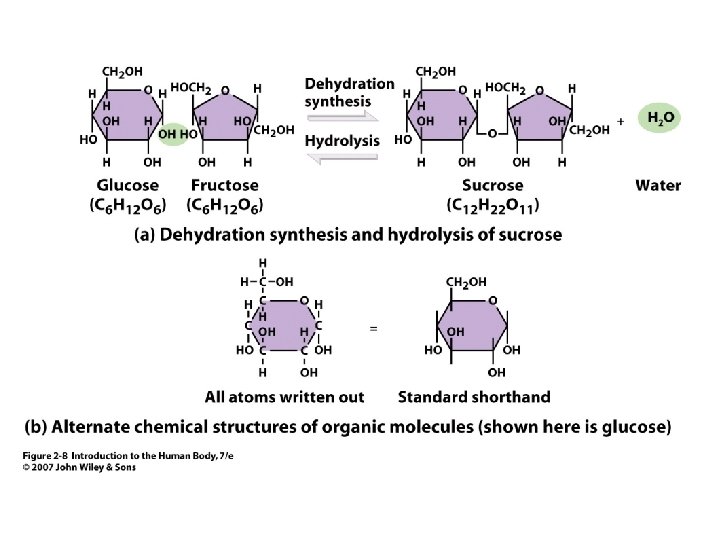
• • Simple sugars = Monosaccharide Major one in body = glucose Disaccharides= 2 - simple sugars bonded Formed by dehydration synthesis E. g. glucose + fructose => sucrose glucose + galactose => lactose Glucose + glucose => maltose 2 Unit 2 Carbohydrates

Figure 2. 8

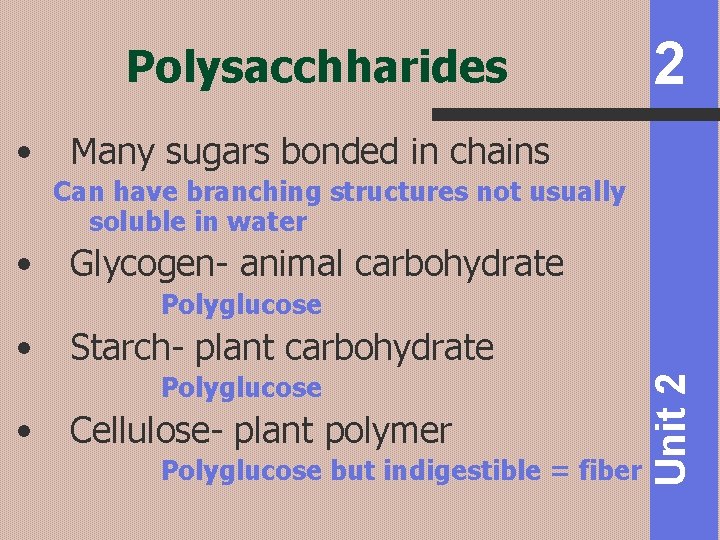
Polysacchharides • 2 Many sugars bonded in chains Can have branching structures not usually soluble in water • Glycogen- animal carbohydrate Polyglucose Starch- plant carbohydrate Polyglucose • Cellulose- plant polymer Polyglucose but indigestible = fiber Unit 2 •

Figure 2. 9

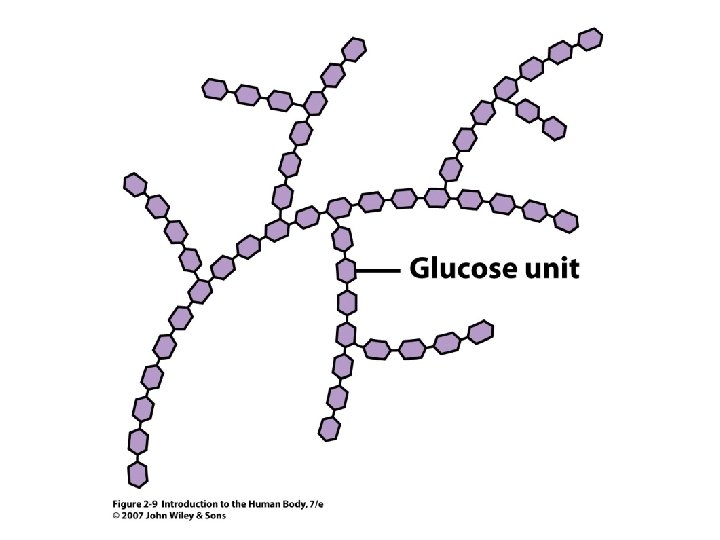
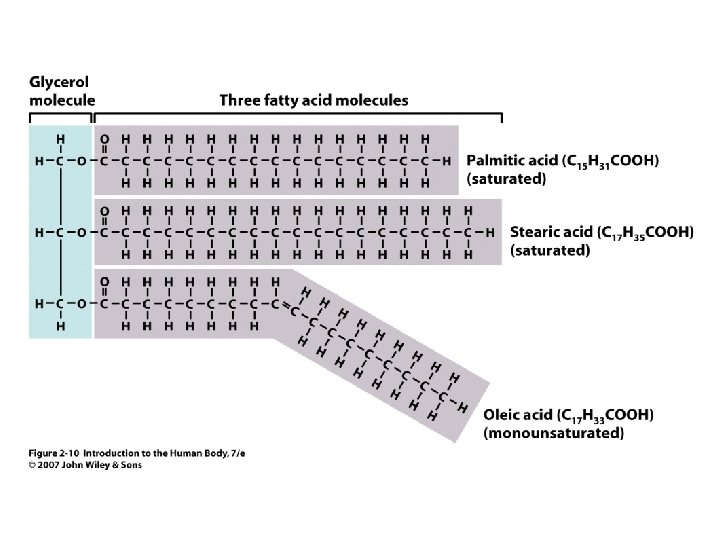
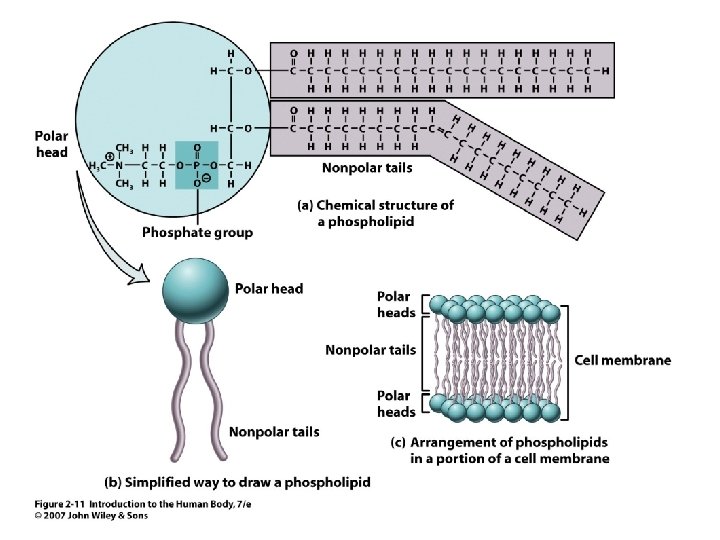
• • Insoluble in water = hydrophobic Triglycerides Phospholipids Cholesterol Steroids Fatty acids Fat soluble vitamins 2 Unit 2 Lipids

Figure 2. 10

Figure 2. 11

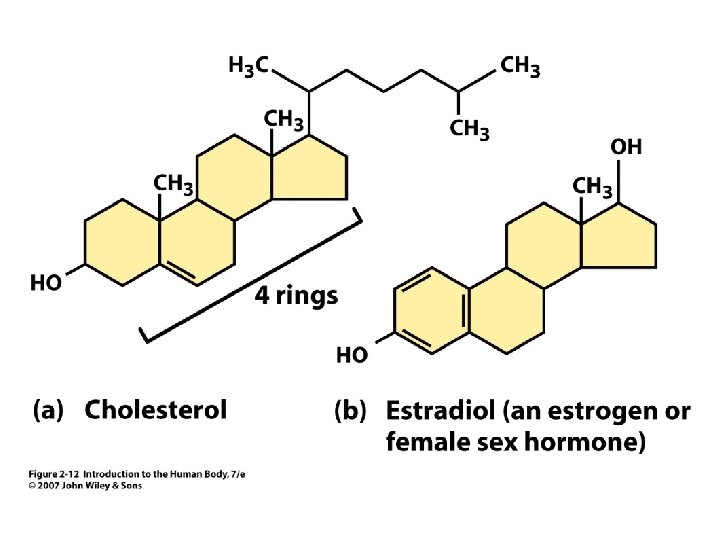
Figure 2. 12

Cholesterol Ring structures Used to make steroid hormones Help make membranes stiff Made in liver Unit 2 • • 2

• • • Structural elements in cells Chemical catalysts Hormones Antibodies Polymers of amino acids 2 Unit 2 Proteins

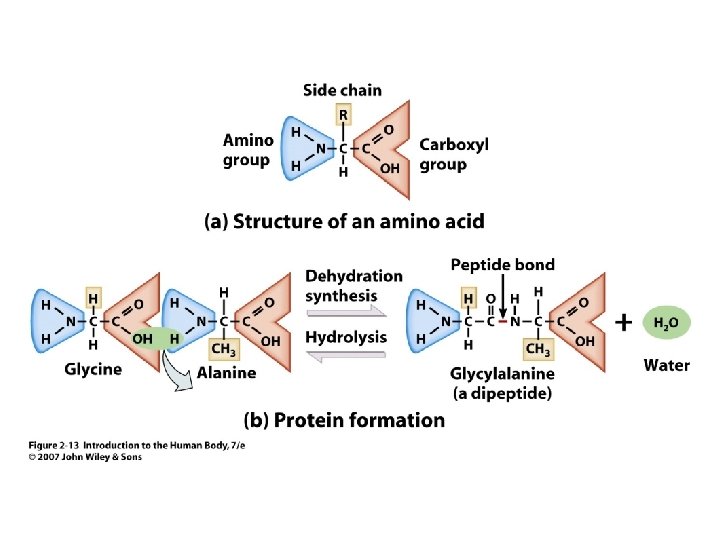
• • • Amino group Carboxyl group Side chain ~20 different side chains A large variety of structures 2 Unit 2 Amino Acid

Figure 2. 13

Terminology Amino acids joined by peptide bond 2 = dipeptide, 3= tripeptide Many =polypeptide Functional polypeptide = protein Includes structure up to quaternary. Thus a protein may have 1 or more polypeptide chains Unit 2 • • 2

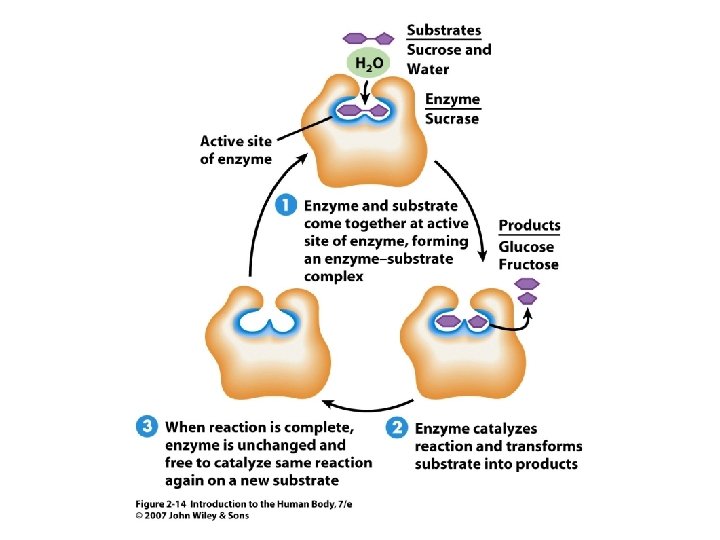
Enzymes • • • Proteins serving as chemical catalysts Highly specific Efficient May be controlled Unit 2 • 2

Figure 2. 14

• • Polymer of nucleotides => Phosphate Sugar –pentose (ribose, deoxyribose) Base- 5 of them (4 per nucleic acid) Adenine (A), guanine (G), thymine (T), cytidine (C), uracil (U) 2 Unit 2 Nucleic Acids

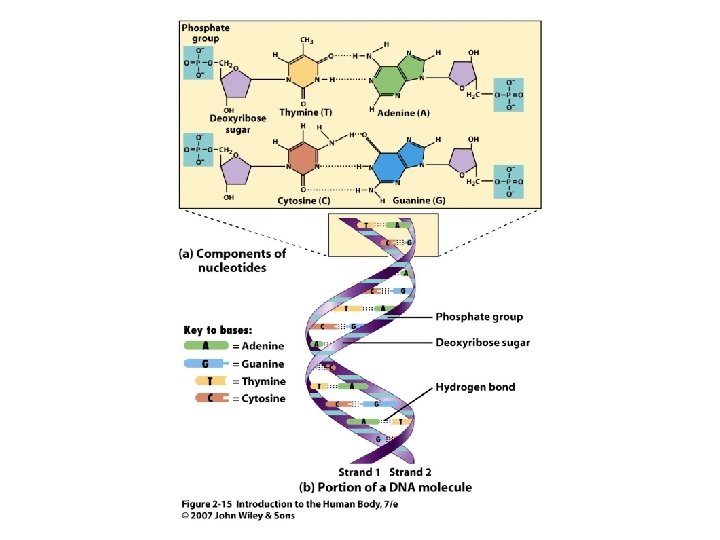
• • • Deoxyribose & A, T, G, C Bases pair: A-T & G-C Two polymers hydrogen bonded together forms a double helix Stores genetic information on protein sequences. 2 Unit 2 DNA

Figure 2. 15

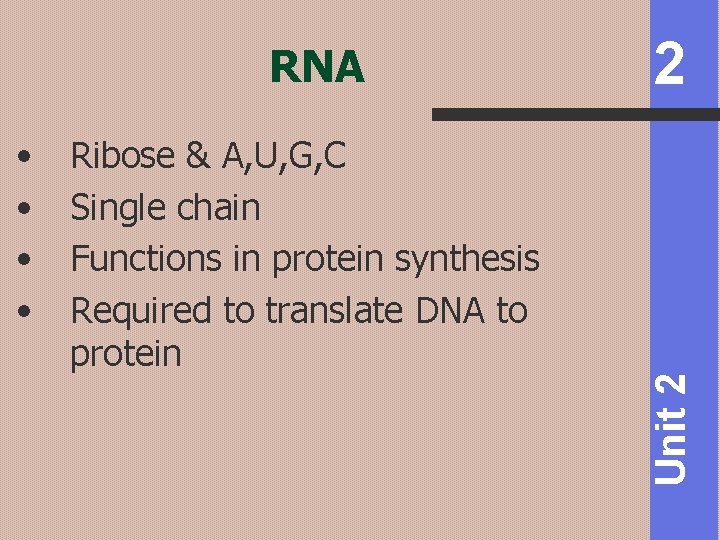
• • Ribose & A, U, G, C Single chain Functions in protein synthesis Required to translate DNA to protein 2 Unit 2 RNA

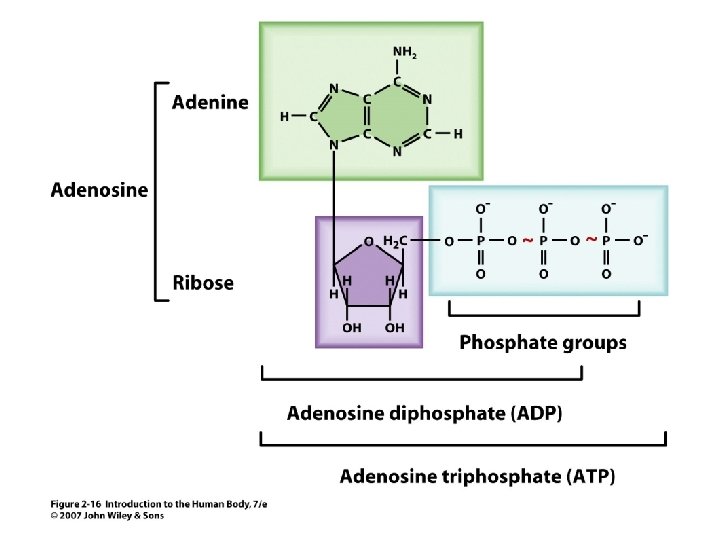
ATP • Specialized for energy transport in the cell Carries energy in the chemical bond between phosphate groups. Unit 2 • 2

Figure 2. 16