Introduction Topic Pure Substances and Mixtures Teaching Points

Introduction • Topic: Pure Substances and Mixtures • Teaching Points: (a) Pure Substances • (i)Elements • (ii)Compounds • (b) Mixtures • Specific Aim : To appreciate the idea about Pure Substances and Mixtures, to know the properties of both Pure Substances and Mixtures, to let the students apply the idea in their practical life.

Some Questions and Answers • • • Question: What is an Atom? Answer: An Atom is the smallest part of an element and has all the properties of that element. Question: What is a Molecule? Answer: The smallest part of an element or a compound that can exist independently is called a Molecule. Question: Name the smallest part of an Element. Answer: The smallest part of an Element is called an Atom.

Some Questions and Answers • • • Question: Name the smallest part of a Compound. Answer: The smallest part of a Compound is called a Molecule. Question: What is a Pure Substance? Answer: Any substance without impurities is known as pure substance. Question: Can we call Elements and Compounds pure substances? Answer: Yes, Elements and Compounds are pure Substance

Introduction • You know cadets; • Any substance without impurities is known as Pure Substance. • A Pure Substance has same type of constituent particles. • Today, we shall discuss about Pure Substances and Mixtures.

Pure Substances & Mixtures: Pure Substances: o The substances which are made of only one kind of atoms or molecules are known as Pure substances. E. g. Elements and Compounds o Properties: Pure Substances have fixed Melting and Boiling Points. Addition of impurities will change the melting and Boiling points of Pure substances. Mixtures: Materials that contain different substances (either Elements, or Elements and Compounds, or just Compounds) physically mixed in any ratio are called Mixtures. o Properties: Mixtures are not pure substances Mixtures do not have fixed Melting and Boiling points.
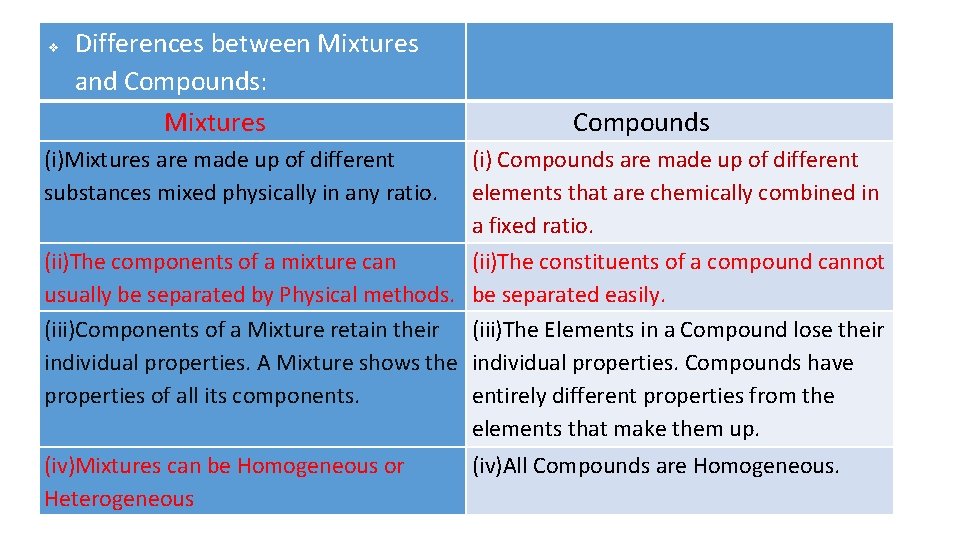
Differences between Mixtures and Compounds: Differences between and Compounds: Mixtures Compounds (i)Mixtures are made up of different substances mixed physically in any ratio. (i) Compounds are made up of different elements that are chemically combined in a fixed ratio. (ii)The components of a mixture can (ii)The constituents of a compound cannot usually be separated by Physical methods. be separated easily. (iii)Components of a Mixture retain their (iii)The Elements in a Compound lose their individual properties. A Mixture shows the individual properties. Compounds have properties of all its components. entirely different properties from the elements that make them up. (iv)Mixtures can be Homogeneous or Heterogeneous (iv)All Compounds are Homogeneous.

Homogeneous Mixture & Heterogeneous Mixture Homogeneous Mixture: The mixture in which the particles of different substances are spread evenly is known as Homogeneous Mixture. Heterogeneous Mixture: The mixture in which the particles of different substances are spread unevenly is known as Heterogeneous Mixture.

Some Questions And Answers • • • Question: What are Pure Substances? Answer: The substances which are made of only one kind of atoms or molecules are known as Pure substances. Question: What are Mixtures? Answer: Materials that contain different substances (either Elements, or Elements and Compounds, or just Compounds) physically mixed in any ratio are called Mixtures. Question: Give the definition of Homogeneous Mixture? Answer: The mixture in which the particles of different substances are spread evenly is known as Homogeneous Mixture.

Questions & Answers • • • Question: What is a Heterogeneous Mixture? Answer: The mixture in which the particles of different substances are spread unevenly is known as Heterogeneous Mixture. Question: Why is it difficult to separate the constituents of a Compound? Answer: It is difficult to separate the constituents of a Compound because constituents are chemically combined in a fixed ratio. Question: Why can the constituents of a Mixture retain their individual properties? Answer: The constituents of a Mixture can retain their individual properties because they are physically mixed in any ratio.
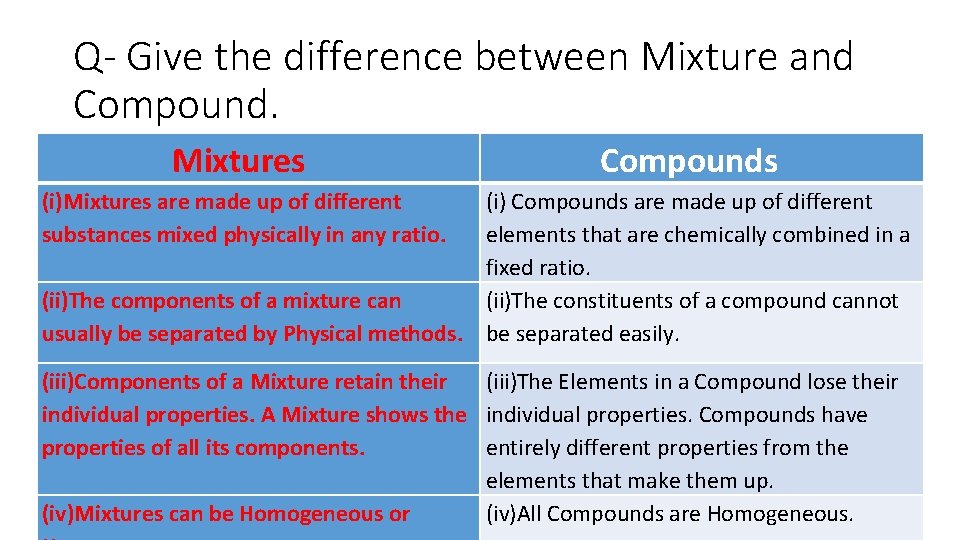
Q- Give the difference between Mixture and Compound. Mixtures Compounds (i)Mixtures are made up of different substances mixed physically in any ratio. (i) Compounds are made up of different elements that are chemically combined in a fixed ratio. (ii)The components of a mixture can (ii)The constituents of a compound cannot usually be separated by Physical methods. be separated easily. (iii)Components of a Mixture retain their (iii)The Elements in a Compound lose their individual properties. A Mixture shows the individual properties. Compounds have properties of all its components. entirely different properties from the elements that make them up. (iv)Mixtures can be Homogeneous or (iv)All Compounds are Homogeneous.
- Slides: 10