Introduction to Water Quality Engineering DRINKING WATER STANDARDS




![p. H- Hydrogen Ion Concentration p. H= log[1/H+] 0 Acidic 7 Alkaline Pure water p. H- Hydrogen Ion Concentration p. H= log[1/H+] 0 Acidic 7 Alkaline Pure water](https://slidetodoc.com/presentation_image_h2/b3e3a579c7b7c0d95b308a08f3884cfa/image-7.jpg)


















- Slides: 51

Introduction to Water Quality Engineering

DRINKING WATER STANDARDS FPrimary Standards, enforeceable by law are parameters that directly affect human health. FSecondary Standards are related to aesthetics and are not enforceable by the federal government.

Classification of Water Quality Parameters Water quality parameters can be classified in a number of ways, but most often are grouped as physical, chemical and biological. l Physical Taste, Odors, Color, Temperature, Turbidity l Chemical Inorganics (Calcium, Magnesium, Iron etc. ) Organics (Pesticides, Herbicides, Petroleum) l Biological Bacteria, Viruses, Algae

Sources of Water n Surface Water - Rivers, Lakes, Streams l High Withdrawal Rates l Polluted l Require Treatment n Ground Water n Sea Water n Reclaimed Wastewater

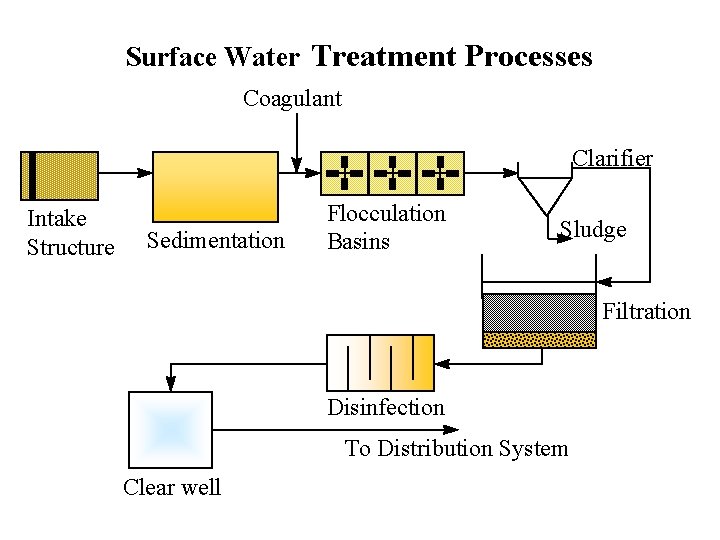
Surface Water Treatment Processes Coagulant Clarifier Intake Structure Sedimentation Flocculation Basins Sludge Filtration Disinfection To Distribution System Clear well

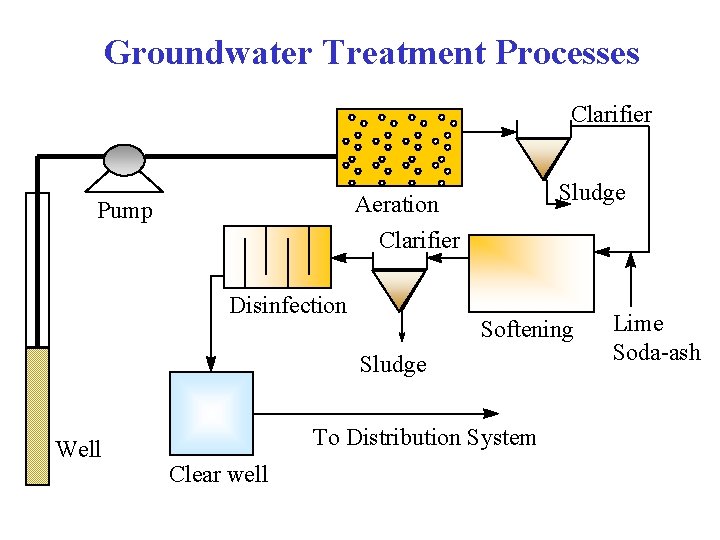
Groundwater Treatment Processes Clarifier Sludge Aeration Clarifier Pump Disinfection Softening Sludge Well To Distribution System Clear well Lime Soda-ash
![p H Hydrogen Ion Concentration p H log1H 0 Acidic 7 Alkaline Pure water p. H- Hydrogen Ion Concentration p. H= log[1/H+] 0 Acidic 7 Alkaline Pure water](https://slidetodoc.com/presentation_image_h2/b3e3a579c7b7c0d95b308a08f3884cfa/image-7.jpg)
p. H- Hydrogen Ion Concentration p. H= log[1/H+] 0 Acidic 7 Alkaline Pure water has a p. H of 7. It is neutral since [H+]=[OH-]=10 -7 mole/L • p. H affects major water treatment processes like lcoagulation lsoftening ldisinfection lcorrosion control 14

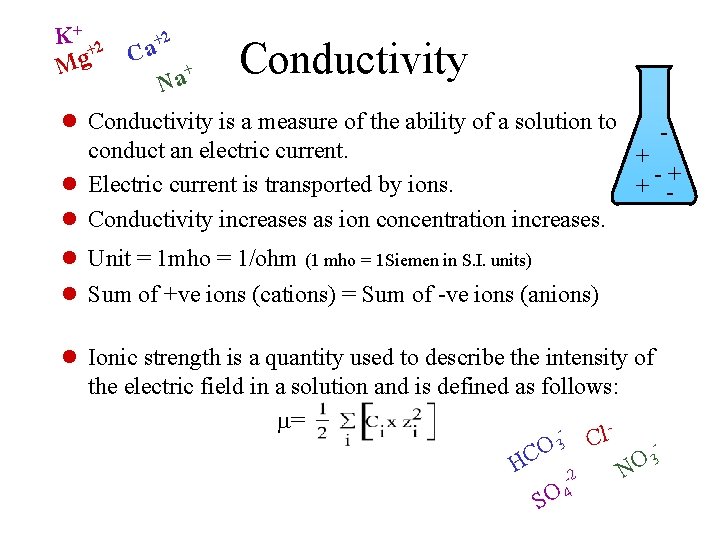
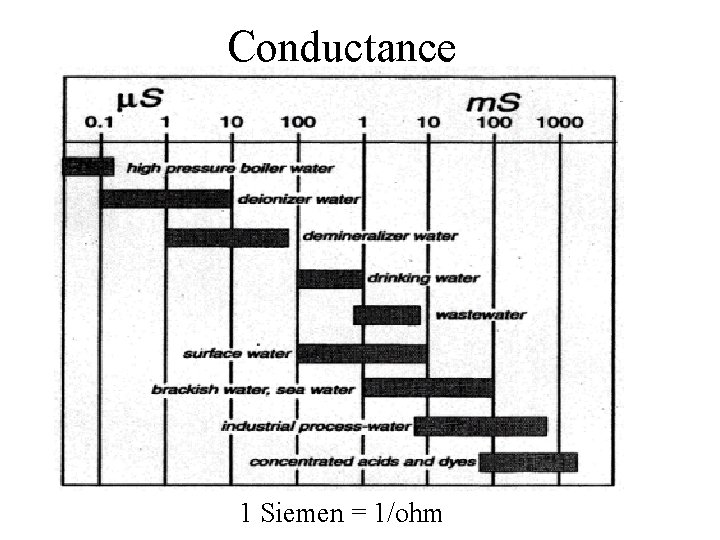
K+ 2 + g M +2 Ca + a N Conductivity l Conductivity is a measure of the ability of a solution to conduct an electric current. + l Electric current is transported by ions. + - -+ l Conductivity increases as ion concentration increases. l Unit = 1 mho = 1/ohm (1 mho = 1 Siemen in S. I. units) l Sum of +ve ions (cations) = Sum of -ve ions (anions) l Ionic strength is a quantity used to describe the intensity of the electric field in a solution and is defined as follows: m= l C O 3 C 3 O H N -2 SO 4

Turbidity and Color • Turbidity - The tendency of water to scatter light at 90 degrees. Turbidity is a measure of water clarity. Caused by suspended solids (thus, turbidity is an indirect measure of suspended solids). • Measured in NTUs, using a Turbidimeter. For most people, water with <= 5 NTUs looks clear. The American Water Works Association (AWWA) recommends that water to be disinfected should be <= 0. 1 NTU. • Color - True color is caused by dissolved compounds in water. It can be natural or anthropocentric. Dissolved and suspended solids (together) cause apparent color. • Color is measured in Platinum-Cobalt units. The AWWA recommends <= 15 Platinum Cobalt units. This is also the U. S. secondary drinking water regulation. Color can be measured using light with a wavelength of 455 nm.

Salinity • Measured mainly as Specific Gravity (SG), and Conductivity (C) by use of a hydrometer or electronic probe. • Saltwater is heavier than freshwater.

Alkalinity • The alkalinity of water is a measure of its capacity to neutralize acids. Three major classes of materials cause the major portion of alkalinity in natural waters. These are as follows: (1) hydroxide (2) carbonates and (3) bicarbonates. Bicarbonates represent the major form of alkalinity.

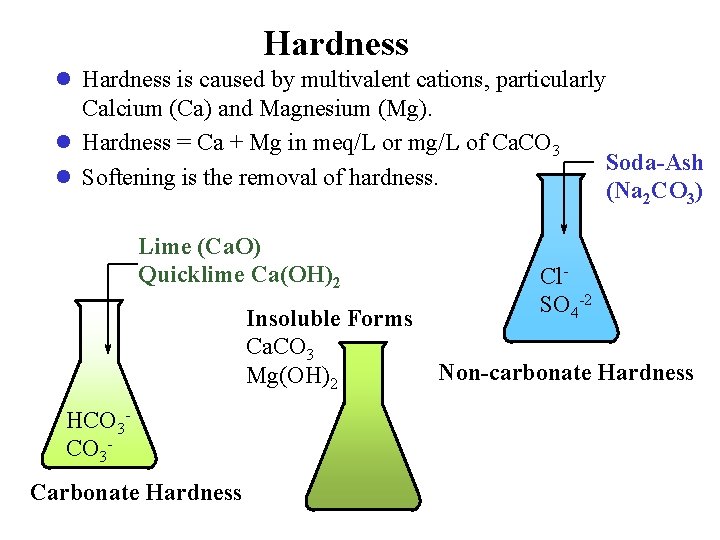
Hardness l Hardness is caused by multivalent cations, particularly Calcium (Ca) and Magnesium (Mg). l Hardness = Ca + Mg in meq/L or mg/L of Ca. CO 3 l Softening is the removal of hardness. Lime (Ca. O) Quicklime Ca(OH)2 Insoluble Forms Ca. CO 3 Mg(OH)2 HCO 3 Carbonate Hardness Soda-Ash (Na 2 CO 3) Cl. SO 4 -2 Non-carbonate Hardness

Nitrate and Phosphate • Nitrate is a primary drinking water standard. Their presence also causes the blue-baby syndrome in infants. Nitrate is also a nutrient for algae and can stimulate growth of algae. • Phosphate is a major source of pollution in surface waters from human activities such as irrigation and agriculture. It is a nutrient for plants and algae and can cause eutrophication of lakes.

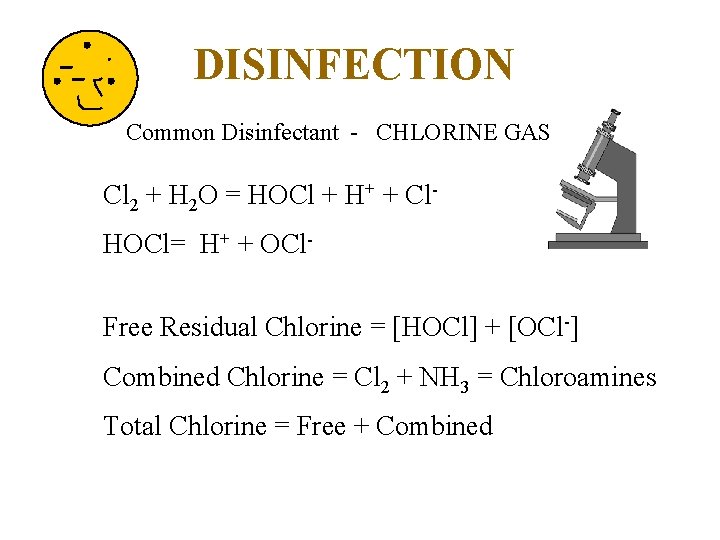
DISINFECTION Common Disinfectant - CHLORINE GAS Cl 2 + H 2 O = HOCl + H+ + Cl. HOCl= H+ + OCl. Free Residual Chlorine = [HOCl] + [OCl-] Combined Chlorine = Cl 2 + NH 3 = Chloroamines Total Chlorine = Free + Combined

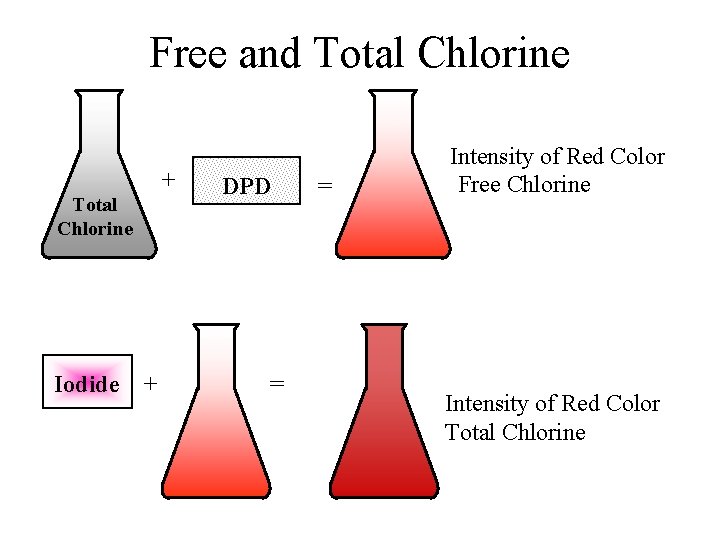
Free and Total Chlorine + Total Chlorine Iodide + DPD = = Intensity of Red Color Free Chlorine Intensity of Red Color Total Chlorine

Freshman Engineering Clinic Understanding Probes and Meters

Overview • • • Conductivity p. H DO Turbidimeter Spectrophotometer

Conductivity Probe

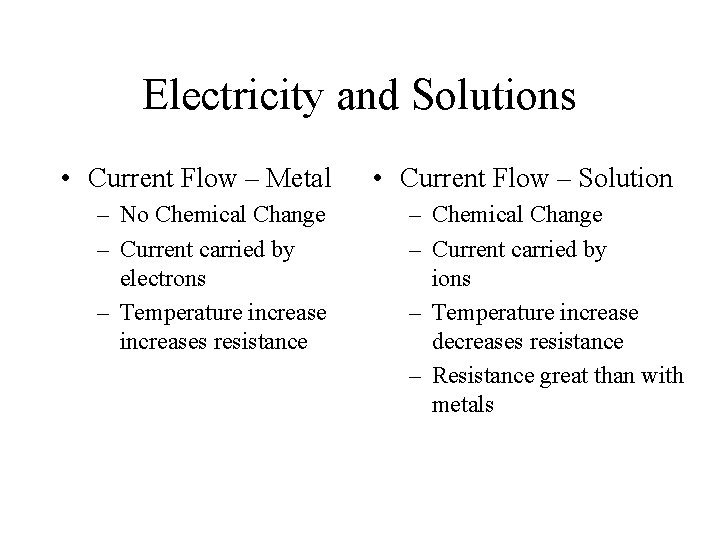
Electricity and Solutions • Current Flow – Metal – No Chemical Change – Current carried by electrons – Temperature increases resistance • Current Flow – Solution – Chemical Change – Current carried by ions – Temperature increase decreases resistance – Resistance great than with metals

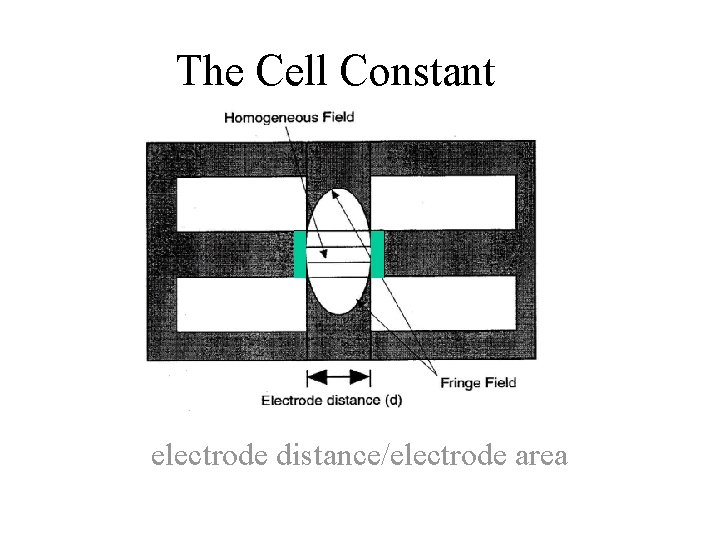
The Cell Constant electrode distance/electrode area

Conductance 1 Siemen = 1/ohm

Typical Ohm Meter

Summary • The Conductivity Meter is made up of a probe which has the two electrodes with a fixed area and a fixed distance apart. • An electric current goes through the solution. • The digital display functions as the signal converter so the user can see the results. • Conductance is simply the inverse of Resistance.

p. H Probe

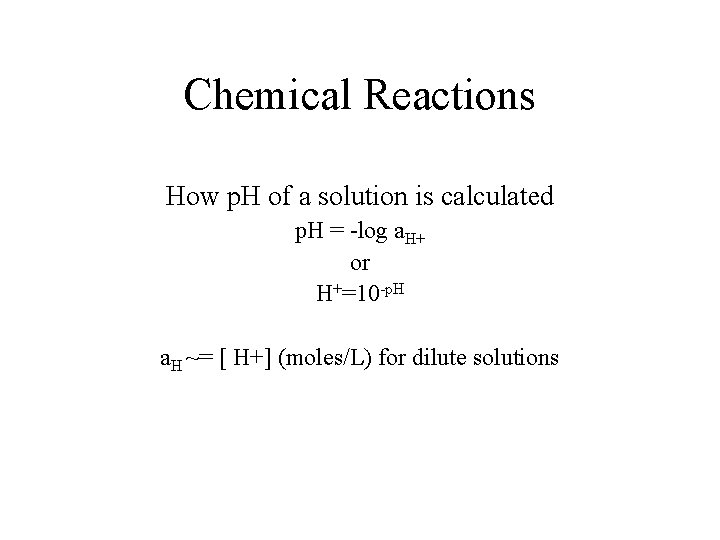
Chemical Reactions How p. H of a solution is calculated p. H = -log a. H+ or H+=10 -p. H a. H ~= [ H+] (moles/L) for dilute solutions

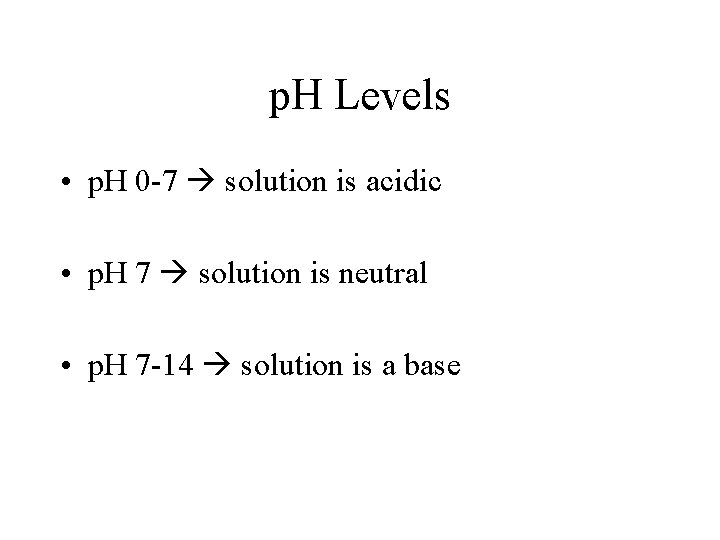
p. H Levels • p. H 0 -7 solution is acidic • p. H 7 solution is neutral • p. H 7 -14 solution is a base

Probe Electrodes or sensing membranes

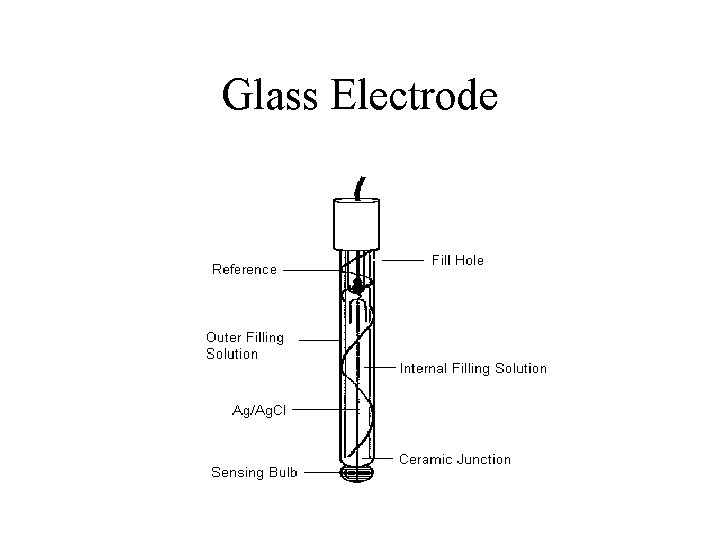
Glass Electrode

Probe Meter

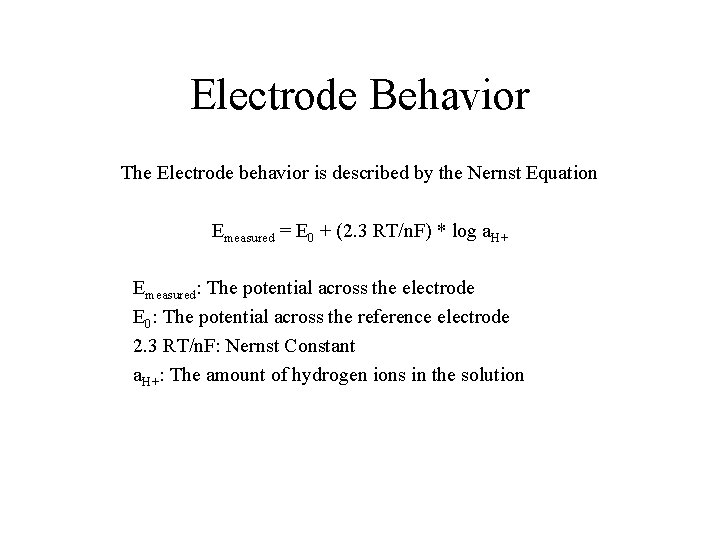
Electrode Behavior The Electrode behavior is described by the Nernst Equation Emeasured = E 0 + (2. 3 RT/n. F) * log a. H+ Emeasured: The potential across the electrode E 0: The potential across the reference electrode 2. 3 RT/n. F: Nernst Constant a. H+: The amount of hydrogen ions in the solution

Summary • A potential develops across the electrode when the probe is inserted in the solution • Comparison between the unvarying potential and the potential across the electrodes • The potential is sent to the meter calibrated from 0 -14 through the wire that connect them • The p. H is calculated by the meter’s software using Nersnt’s equation.

Dissolved Oxygen Probe

Two Types of DO Probes • Galvanic • Polarographic

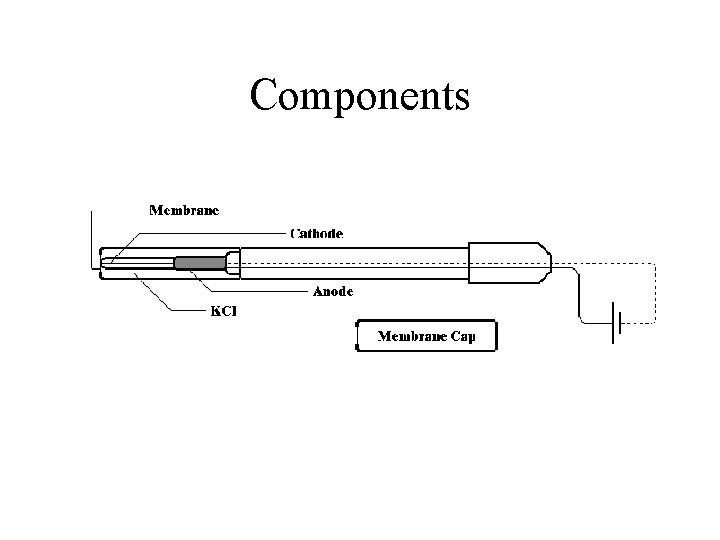
Components

DO Probe Characteristics • The p. H of the solution does not affect the performance of DO probes. • Chlorine and hydrogen sulfide cause erroneous readings in DO probes. • Atmospheric pressure affects the saturation of oxygen. DO probes must be calibrated for the barometric pressure when reading in mg/l. • Membrane thickness determines the output level of the probe. • Membrane thickness also determines the speed of response to change in DO levels.

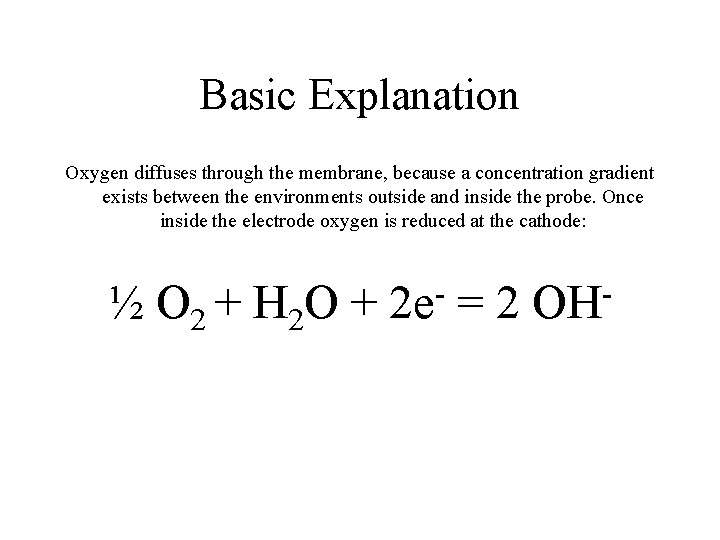
Basic Explanation Oxygen diffuses through the membrane, because a concentration gradient exists between the environments outside and inside the probe. Once inside the electrode oxygen is reduced at the cathode: ½ O 2 + H 2 O + 2 e =2 OH

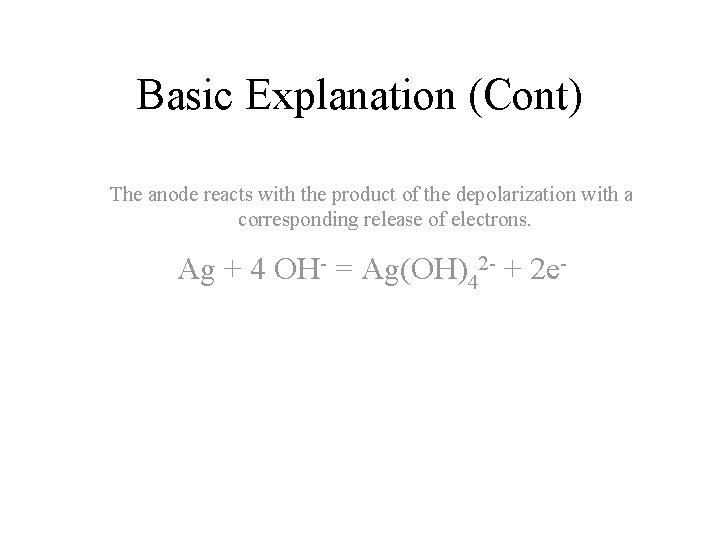
Basic Explanation (Cont) The anode reacts with the product of the depolarization with a corresponding release of electrons. Ag + 4 OH- = Ag(OH)42 - + 2 e-

Summary • Oxygen diffuses through the membrane on to the cathode. • Here it has a chemical reaction and combines with the anode. • This causes an electrical current, which is converted to voltage by flowing across a resistor. • Then this voltage is sent to a meter where the result is analysed and a corresponding DO value is displayed.

Turbidimeter

Turbidity • What is it? – Clarity of water – Measured in Nephelometric Turbidity Units (NTU’s)

Cause of Turbidity • Presence of clay, silt, organic matter, and soil erosion • Scattering of light increases with a greater presence of these materials in water

Hach 2100 N Laboratory Turbidimeter

Turbidimeter • Micro-processor-based model • Employs advanced optical and electronic design • Has two types of sensors – Scattering Sensor – Transmissive Sensor

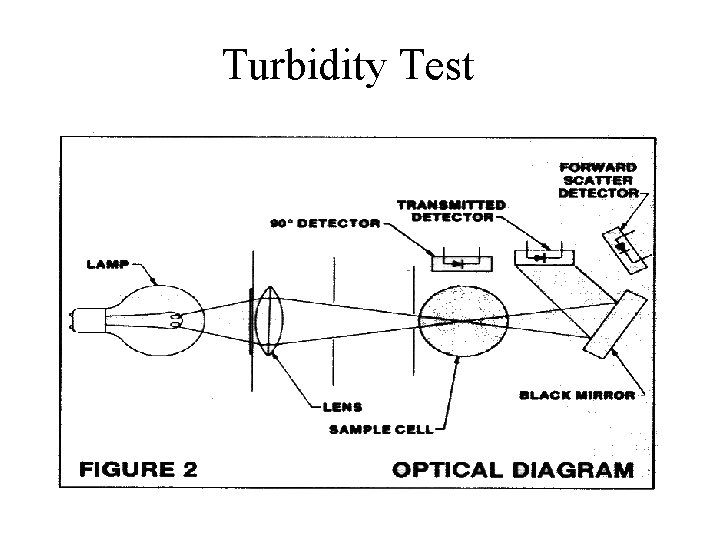
Turbidity Test

Summary • • Turbidity is the clarity of water We measure turbidity in NTU’s Particles suspended in water cause turbidity In the lab, we use the Hach 2100 N Laboratory turbidemeter to measure turbidity • The scattering of light at a 90 degree angle to the light source is measured.

Spectrophotometer

Spectrophotometry • Most widely used method of quantitive and qualitive analysis in chemical and biological sciences. • Accurate and very sensitive method which can analyze quantities as small as micrograms. • Method depends on the light absorbing properties of either substance being analyzed or a derivative. • Basis is simple: The intensity of light which is transmitted through a solution containing an absorbing substance (chromogen) is decreased by that fraction which is absorbed, and this fraction can be detected photoelectrically.

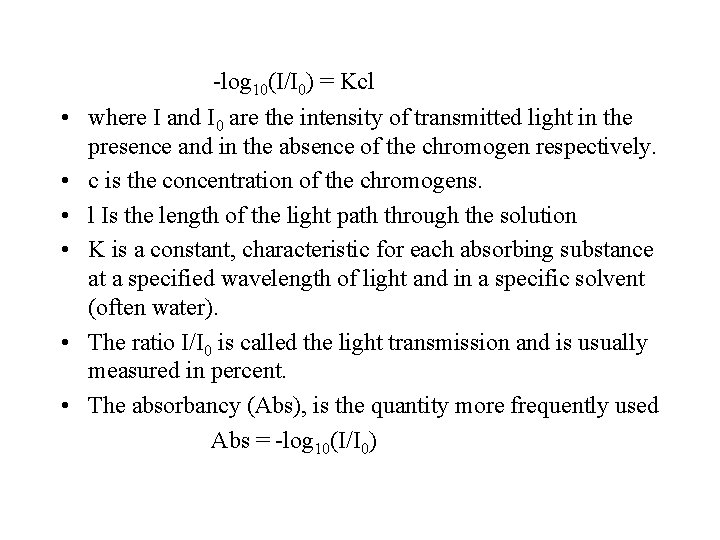
Beer-Lambert law • States that the amount of light absorbed is proportional to the number of molecules of absorbing substance in the light path. • Absorbtion is both proportional to the concentration of the chromogen in a solution and to the length of the light path through the solution. This relationship can be expressed as follows: -log 10(I/I 0) = Kcl

-log 10(I/I 0) = Kcl • where I and I 0 are the intensity of transmitted light in the presence and in the absence of the chromogen respectively. • c is the concentration of the chromogens. • l Is the length of the light path through the solution • K is a constant, characteristic for each absorbing substance at a specified wavelength of light and in a specific solvent (often water). • The ratio I/I 0 is called the light transmission and is usually measured in percent. • The absorbancy (Abs), is the quantity more frequently used Abs = -log 10(I/I 0)

Spectrophotometers • Detects the difference in light through a solution photoelectrically and compares that difference electronically. • The difference is expressed as percent transmission or absorbance.

• In Beer-Lambert law, proportionally constant K depends on: – wavelength of light – nature of absorbing substance • Device uses cells or cuvettes – carefully made solution containers – plane, parallel sides to ensure the light path to be the same through all portions, making it possible to tabulate values of K for various substances in various solvents and wavelengths