Introduction to the Metric System History l Created






- Slides: 22

Introduction to the Metric System

History l Created during French Revolution in 1790 l French King overthrown l National Assembly of France sets up new government l French Academy of Science told to design new system of weights and measures l Lavaiosie appointed to head committee l

History l Called l Systeme International d’Unitès, or SI - International System of Units l Revised l periodically by International Bureau of Weight and Measures

Customary Units of Measurement l The English System l a collection of functionally unrelated units l l l Difficult to convert from one unit to another Ex. 1 ft = 12 inches = 0. 33 yard = 1/5280 miles Customary Units l l length - inch, foot, yard, mile weight/mass - ounce, pound volume - teaspoon, cup, quart, gallon temperature - degrees Fahrenheit time - minutes, hours

Advantages of Using the Metric System l Universal - used everywhere by all scientists to communicate l by all industrialized nations l except United States l U. S. loses billions of dollars in trade l

Advantages of Using the Metric System l Simple l to use A few base units make up all measurements length - meter l mass - grams l volume - liters l temperature – degrees Celsius l time - seconds l

Advantages of Using the Metric System l There is only one unit of measurement for each type of quantity l To simplify things, very small and very large numbers are expressed as multiples of the base unit. l l Prefixes are used to represent how much smaller or larger the quantity is compared to the base unit. Easy to convert from one unit to another l l shift decimal point right shift decimal point left

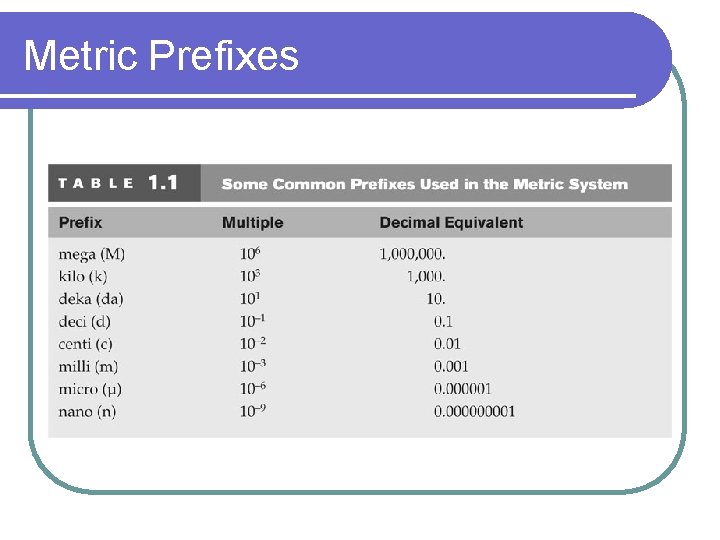
Advantages of Using the Metric System l Same set of prefixes for all units l Greek - multiples of the base l l Latin - fractions of the base l l kilo - 1000 × the base hecto - 100 × the base deka - 10 × the base deci - tenths of the base centi - hundredths of the base milli - thousandths of the base Mnemonic: “Kids Have Dropped Over Dead Converting Metrics. ”

Metric Prefixes

Units of Measurement l Length - the distance between two points standard unit is meter (m) l long distances are measured in km l l l 1 km = 1000 m, 1 m = 1/1000 th km Small distances measured in cm or mm 1 m = 100 cm , 1 cm = 1/100 th m l 1 m = 1000 mm, 1 mm = 1/1000 th m l 10 mm = 1 cm l l Measured using a meter stick or ruler

Units of Measurement l Mass l - the quantity of matter in an object standard unit is gram (g) l Measured using a digital scale or triple beam balance

Units of Measurement l Volume - the amount of space occupied by an object standard unit is liter (L) l 1 L = 1000 ml = 1000 cm 3 = 1 dm 3 l Measured using a graduated cylinder l

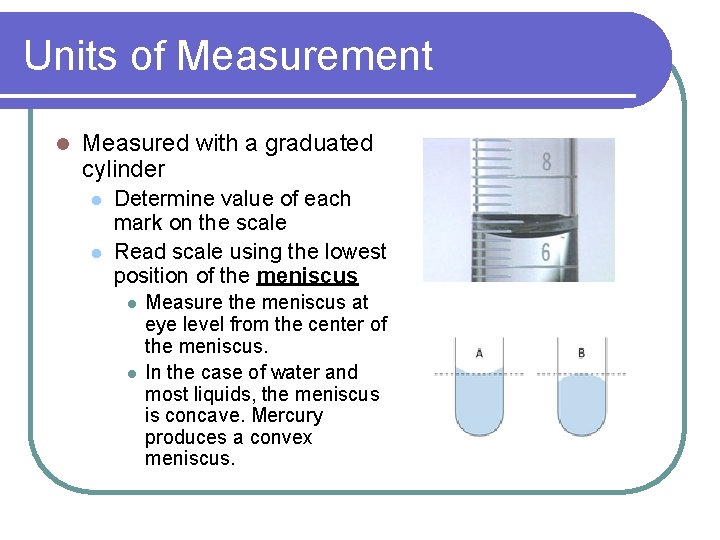
Units of Measurement l Measured with a graduated cylinder l l Determine value of each mark on the scale Read scale using the lowest position of the meniscus l l Measure the meniscus at eye level from the center of the meniscus. In the case of water and most liquids, the meniscus is concave. Mercury produces a convex meniscus.

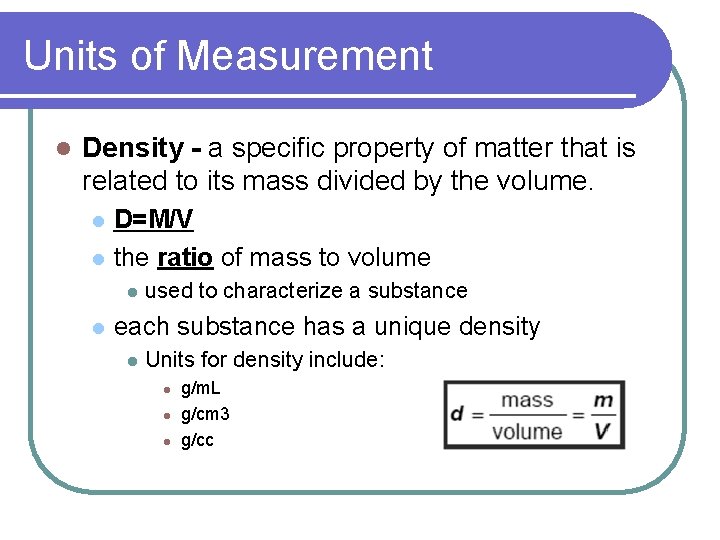
Units of Measurement l Density - a specific property of matter that is related to its mass divided by the volume. l l D=M/V the ratio of mass to volume l l used to characterize a substance each substance has a unique density l Units for density include: l l l g/m. L g/cm 3 g/cc

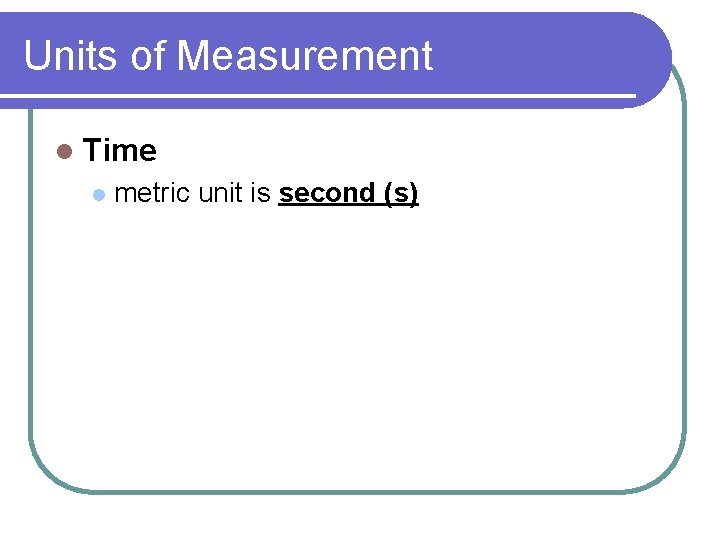
Units of Measurement l Time l metric unit is second (s)

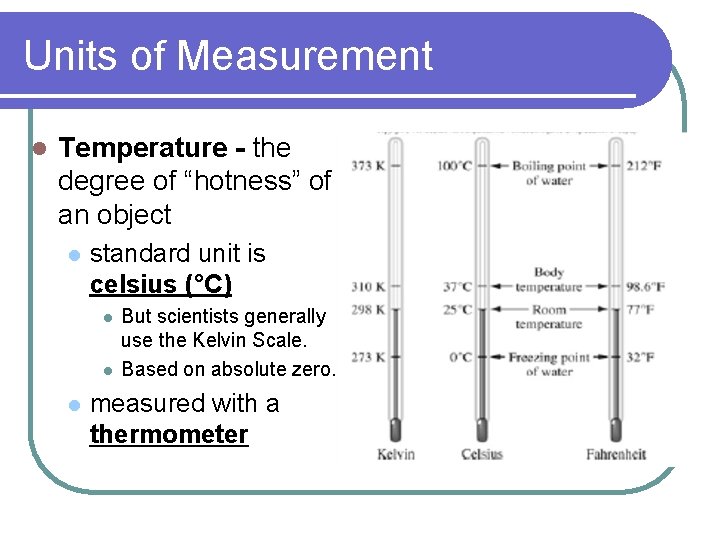
Units of Measurement l Temperature - the degree of “hotness” of an object l standard unit is celsius (°C) l l l But scientists generally use the Kelvin Scale. Based on absolute zero. measured with a thermometer

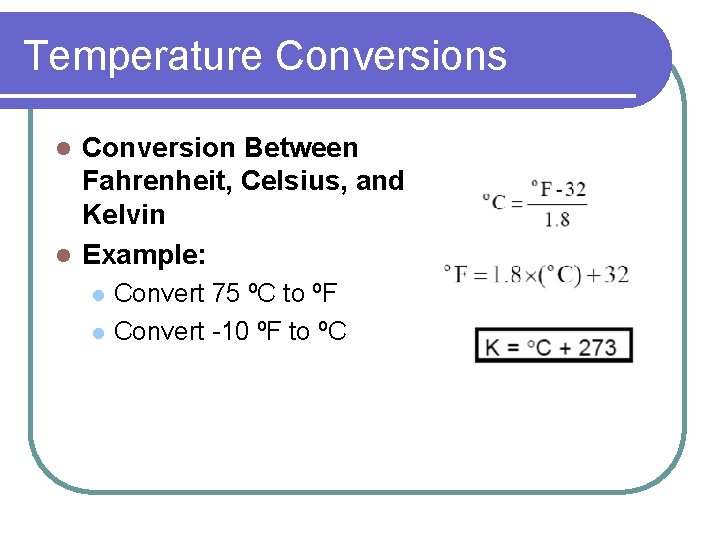
Temperature Conversions Conversion Between Fahrenheit, Celsius, and Kelvin l Example: l l l Convert 75 ºC to ºF Convert -10 ºF to ºC

Conversion and the Metric System

Measurement Unit Conversion l You can convert between units of measurement within the metric system l between the English system and metric system l

Unit Conversion l Let your units do the work for you by simply memorizing connections between units. l l l Example: How many donuts are in one dozen? We say: “Twelve donuts in a dozen. ” Or: 12 donuts = 1 dozen donuts What does any number divided by itself equal? ONE!

Unit Conversion l This fraction is called a unit factor l l Multiplication by a unit factor does not change the amount - only the unit. Example: How many donuts are in 3. 5 dozen? l You can probably do this in your head but try it using the Factor-Label Method.

Unit Conversion Rules Start with the given information… l Then set up your unit factor… l See that the original unit cancels out… l Then multiply and divide all numbers… l