Introduction to Stoichiometry How are mol to mol



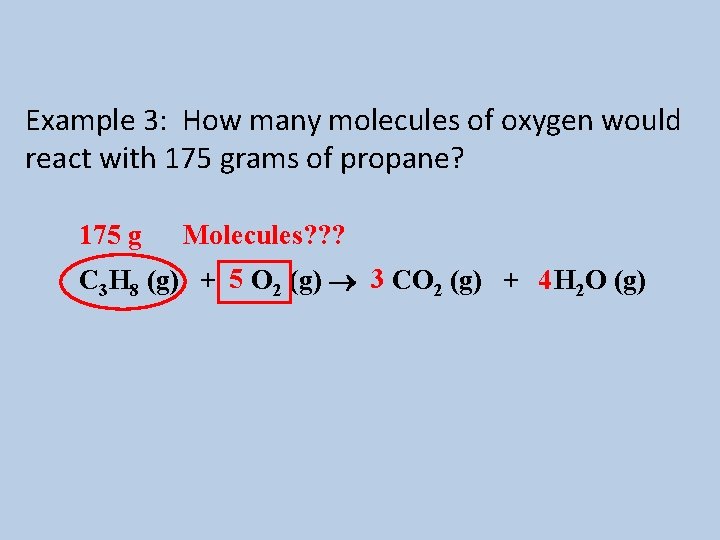

- Slides: 23

Introduction to Stoichiometry How are mol to mol and gram to gram conversion calculated for different elements/compounds in a chemical rxn?

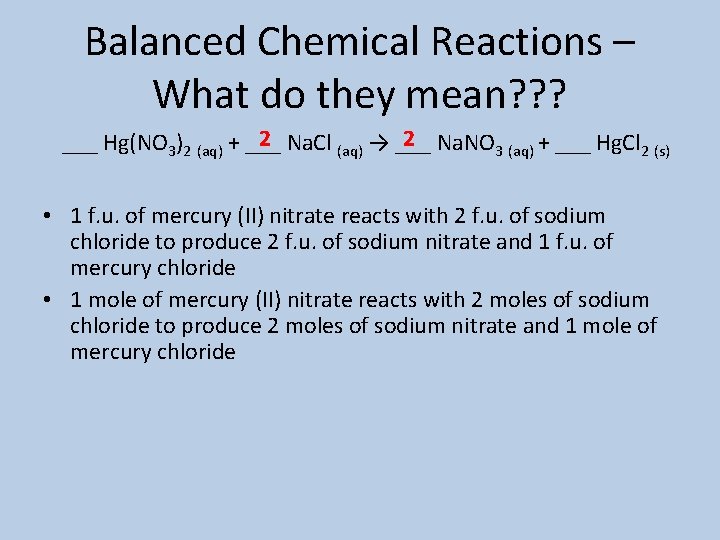
Balanced Chemical Reactions – What do they mean? ? ? 2 Na. Cl (aq) → ___ 2 Na. NO 3 (aq) + ___ Hg. Cl 2 (s) ___ Hg(NO 3)2 (aq) + ___ • 1 f. u. of mercury (II) nitrate reacts with 2 f. u. of sodium chloride to produce 2 f. u. of sodium nitrate and 1 f. u. of mercury chloride • 1 mole of mercury (II) nitrate reacts with 2 moles of sodium chloride to produce 2 moles of sodium nitrate and 1 mole of mercury chloride

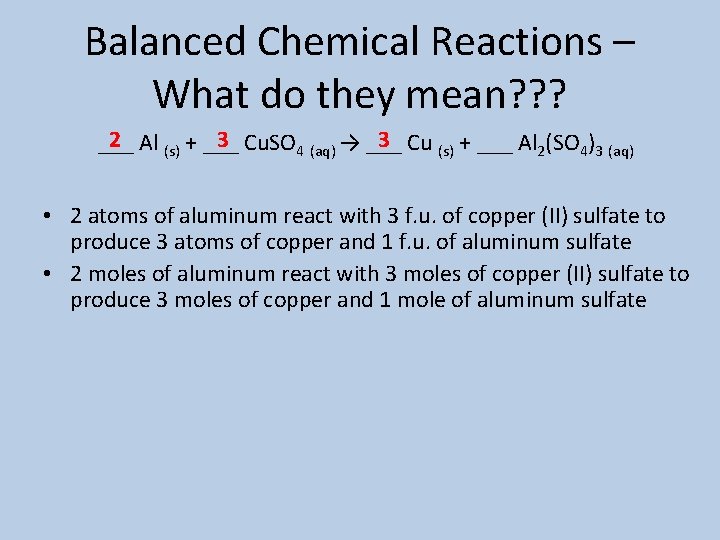
Balanced Chemical Reactions – What do they mean? ? ? 2 Al (s) + ___ 3 Cu. SO 4 (aq) → ___ 3 Cu (s) + ___ Al 2(SO 4)3 (aq) ___ • 2 atoms of aluminum react with 3 f. u. of copper (II) sulfate to produce 3 atoms of copper and 1 f. u. of aluminum sulfate • 2 moles of aluminum react with 3 moles of copper (II) sulfate to produce 3 moles of copper and 1 mole of aluminum sulfate

What is Stoichiometry? • The quantitative relationships between amounts of reactants used and products formed by a chemical reaction. • Shows how much reactant is needed to produce each product • Coefficients are the number of each compound needed or produced in the rxn.

Just like a recipe…. 1 Banana + 5 Strawberries 2 Smoothies How many smoothies can be made from 2 bananas if you had plenty of strawberries? 4, ratio is 1: 2 How many smoothies can be made from 25 strawberries if you had plenty of bananas? 10, ratio is 5: 2

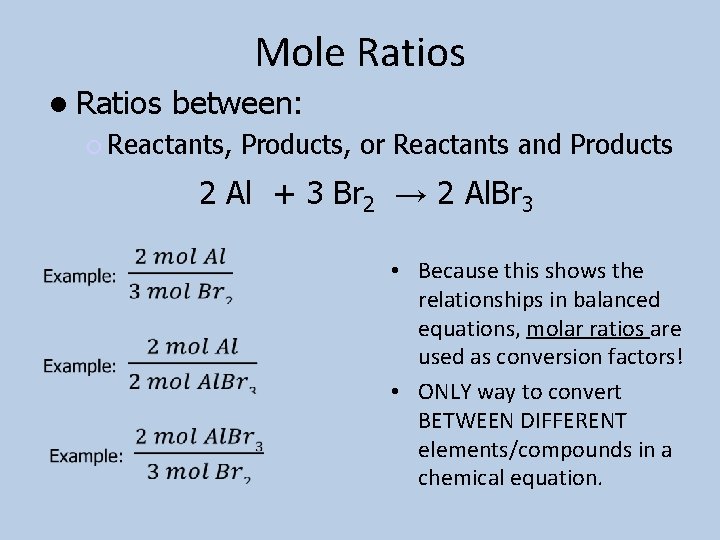
Mole Ratios between: Reactants, Products, or Reactants and Products 2 Al + 3 Br 2 → 2 Al. Br 3 • Because this shows the relationships in balanced equations, molar ratios are used as conversion factors! • ONLY way to convert BETWEEN DIFFERENT elements/compounds in a chemical equation.

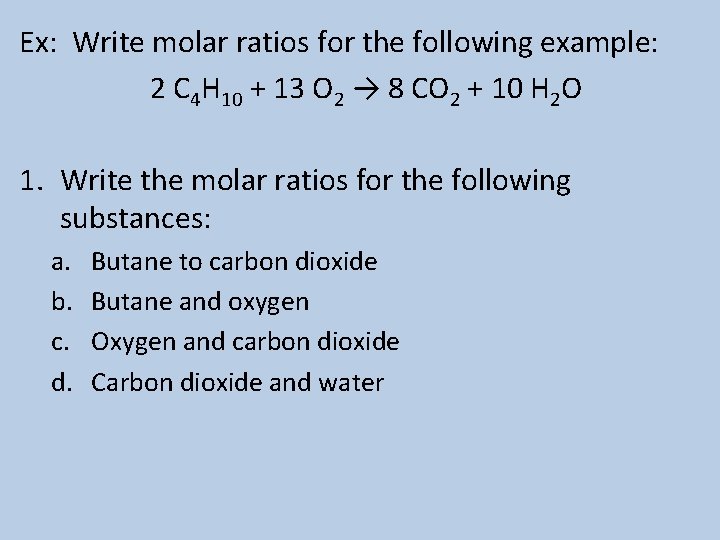
Ex: Write molar ratios for the following example: 2 C 4 H 10 + 13 O 2 → 8 CO 2 + 10 H 2 O 1. Write the molar ratios for the following substances: a. b. c. d. Butane to carbon dioxide Butane and oxygen Oxygen and carbon dioxide Carbon dioxide and water

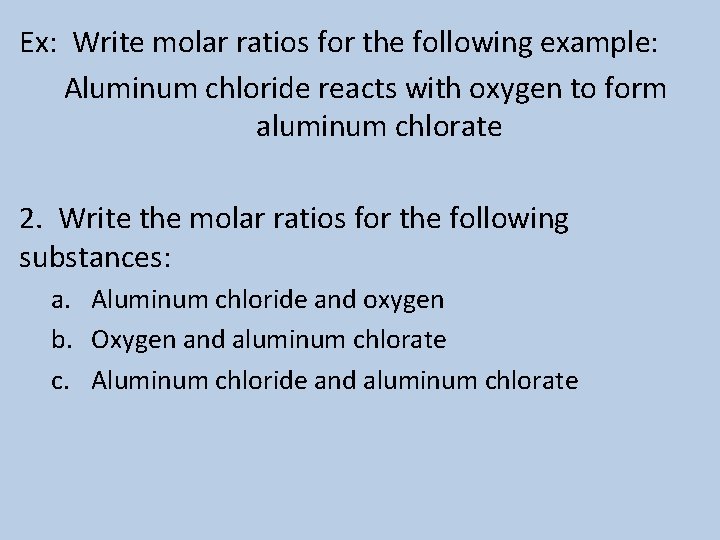
Ex: Write molar ratios for the following example: Aluminum chloride reacts with oxygen to form aluminum chlorate 2. Write the molar ratios for the following substances: a. Aluminum chloride and oxygen b. Oxygen and aluminum chlorate c. Aluminum chloride and aluminum chlorate

Mole to Mole Conversions • Use dimensional analysis to convert from one substance to another substance in a chemical reaction • Helps to determine how much you need to start with or how much you would end up with

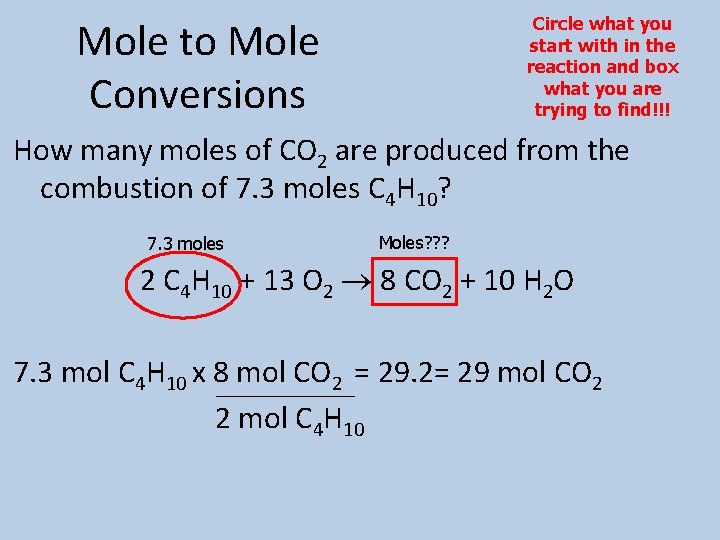
Circle what you start with in the reaction and box what you are trying to find!!! Mole to Mole Conversions How many moles of CO 2 are produced from the combustion of 7. 3 moles C 4 H 10? 7. 3 moles Moles? ? ? 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O 7. 3 mol C 4 H 10 x 8 mol CO 2 = 29. 2= 29 mol CO 2 2 mol C 4 H 10

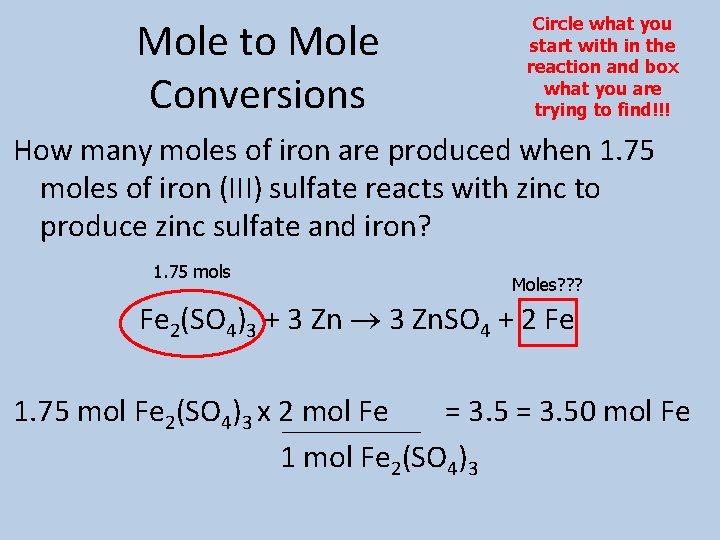
Mole to Mole Conversions Circle what you start with in the reaction and box what you are trying to find!!! How many moles of iron are produced when 1. 75 moles of iron (III) sulfate reacts with zinc to produce zinc sulfate and iron? 1. 75 mols Moles? ? ? Fe 2(SO 4)3 + 3 Zn. SO 4 + 2 Fe 1. 75 mol Fe 2(SO 4)3 x 2 mol Fe = 3. 50 mol Fe 1 mol Fe 2(SO 4)3

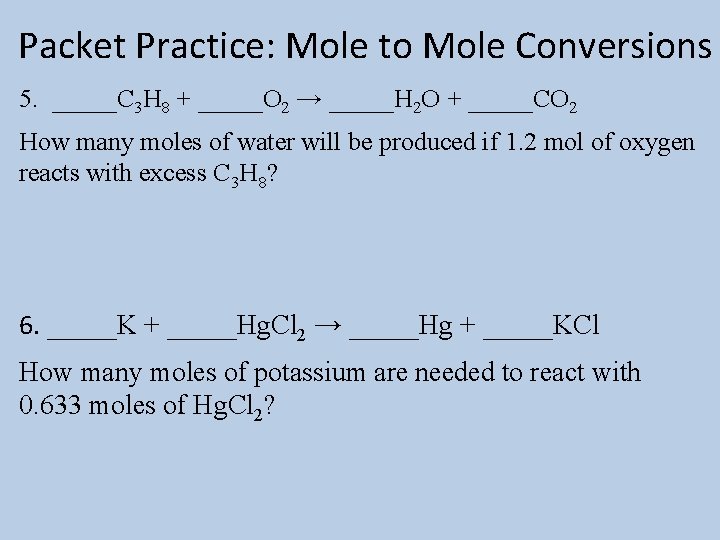
Packet Practice: Mole to Mole Conversions 5. _____C 3 H 8 + _____O 2 → _____H 2 O + _____CO 2 How many moles of water will be produced if 1. 2 mol of oxygen reacts with excess C 3 H 8? 6. _____K + _____Hg. Cl 2 → _____Hg + _____KCl How many moles of potassium are needed to react with 0. 633 moles of Hg. Cl 2?

Gram to Gram Conversions • Used to determine the grams needed or produced in a chemical rxn given the grams of another element/compounds in the rxn. • Conversion factors needed: – 1 mole = molar mass (g) • This will be used twice – Mole ratio • Used to convert between compounds in the chemical rxn

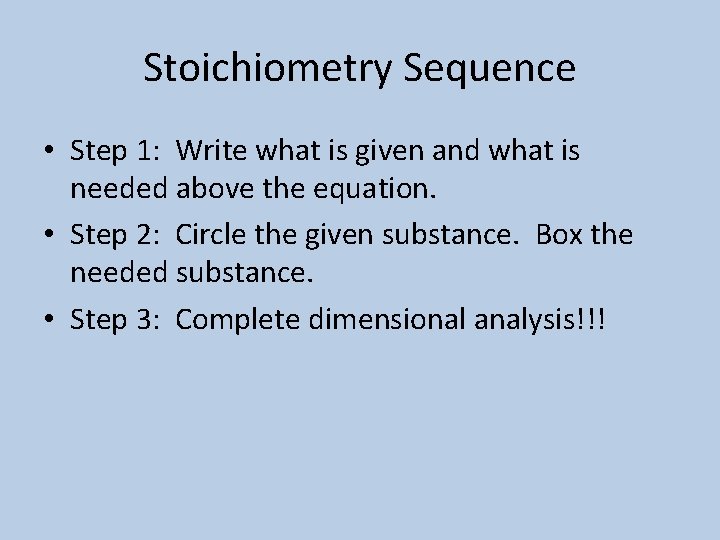
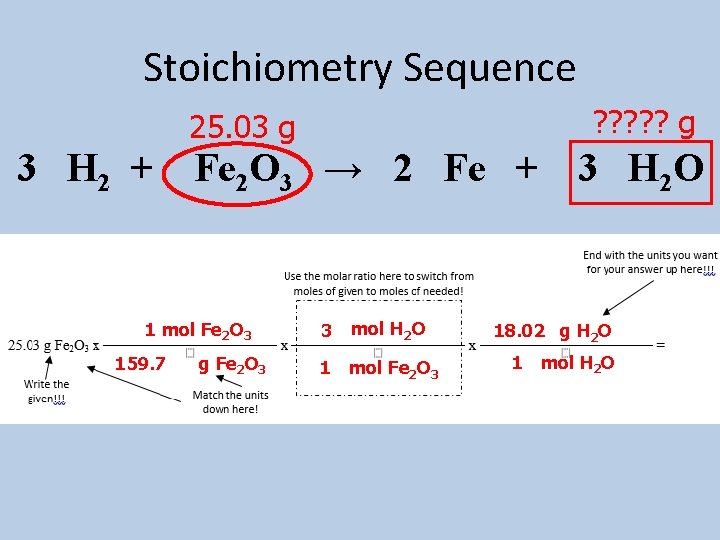
Stoichiometry Sequence • Step 1: Write what is given and what is needed above the equation. • Step 2: Circle the given substance. Box the needed substance. • Step 3: Complete dimensional analysis!!!

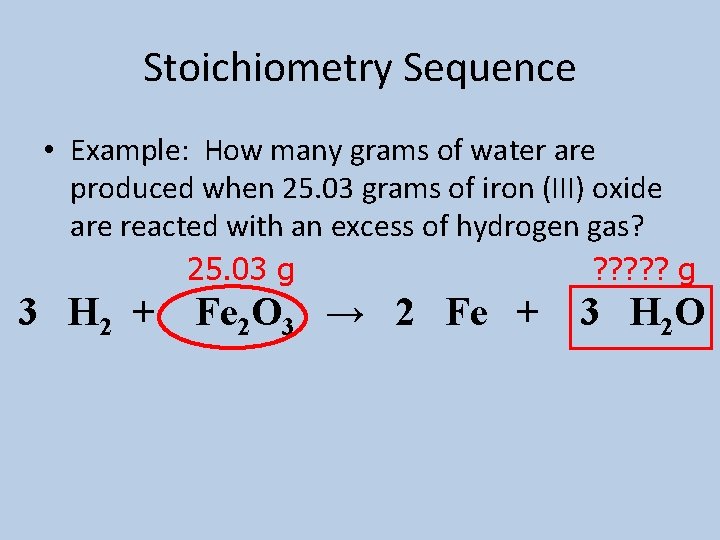
Stoichiometry Sequence • Example: How many grams of water are produced when 25. 03 grams of iron (III) oxide are reacted with an excess of hydrogen gas? 25. 03 g ? ? ? g 3 H 2 + Fe 2 O 3 → 2 Fe + 3 H 2 O

Stoichiometry Sequence ? ? ? g 25. 03 g 3 H 2 + Fe 2 O 3 → 2 Fe + 1 mol Fe 2 O 3 159. 7 g Fe 2 O 3 3 mol H 2 O 1 mol Fe 2 O 3 3 H 2 O 18. 02 g H 2 O 1 mol H 2 O

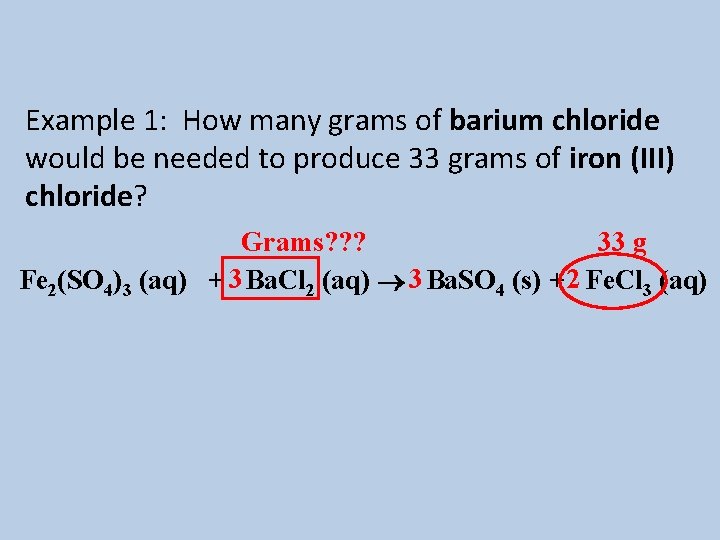
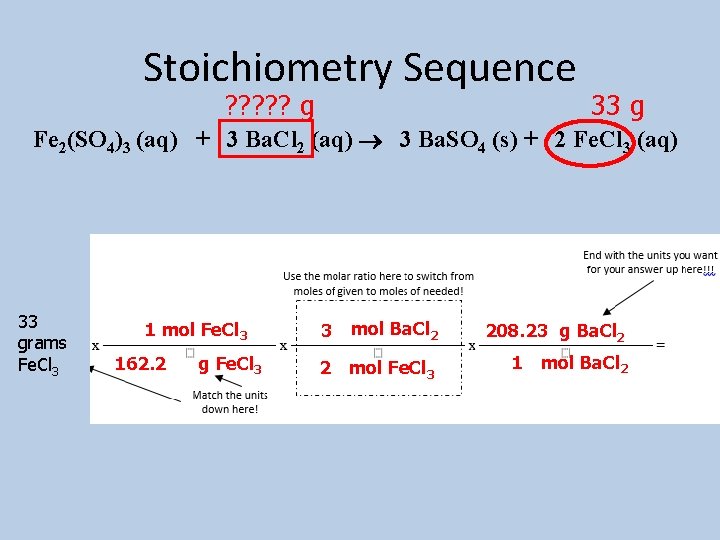
Example 1: How many grams of barium chloride would be needed to produce 33 grams of iron (III) chloride? Grams? ? ? 33 g Fe 2(SO 4)3 (aq) + 3 Ba. Cl 2 (aq) 3 Ba. SO 4 (s) + 2 Fe. Cl 3 (aq)

Stoichiometry Sequence ? ? ? g 33 g Fe 2(SO 4)3 (aq) + 3 Ba. Cl 2 (aq) 3 Ba. SO 4 (s) + 2 Fe. Cl 3 (aq) 33 grams Fe. Cl 3 1 mol Fe. Cl 3 162. 2 g Fe. Cl 3 mol Ba. Cl 2 208. 23 g Ba. Cl 2 2 mol Fe. Cl 3 1 mol Ba. Cl 2 3

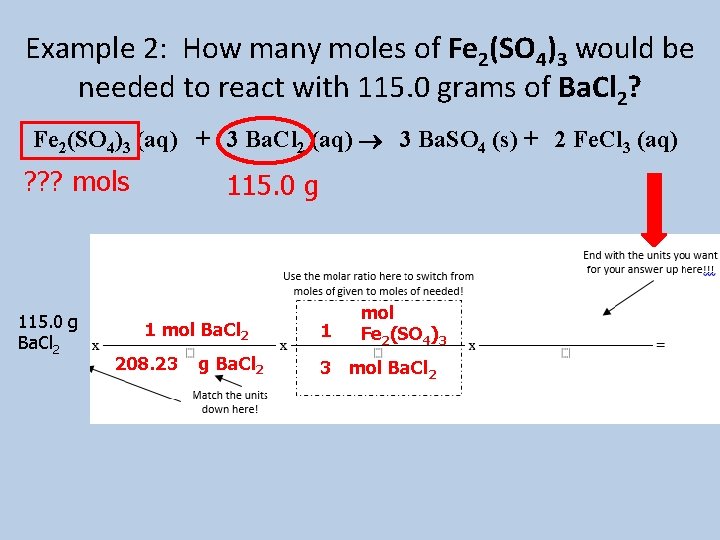
Example 2: How many moles of Fe 2(SO 4)3 would be needed to react with 115. 0 grams of Ba. Cl 2? Fe 2(SO 4)3 (aq) + 3 Ba. Cl 2 (aq) 3 Ba. SO 4 (s) + 2 Fe. Cl 3 (aq) ? ? ? mols 115. 0 g Ba. Cl 2 115. 0 g 1 mol Ba. Cl 2 208. 23 g Ba. Cl 2 1 mol Fe 2(SO 4)3 3 mol Ba. Cl 2
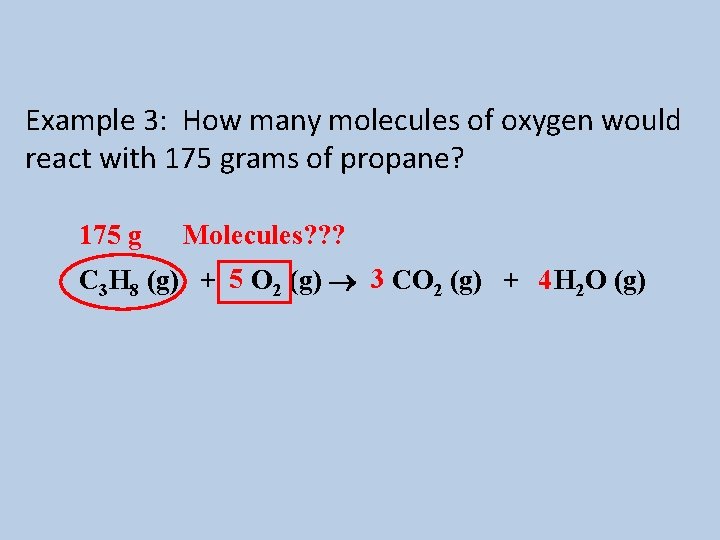
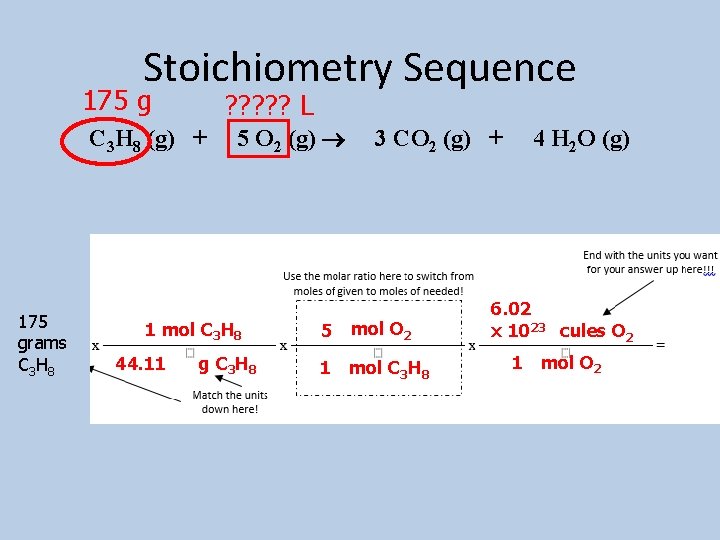
Example 3: How many molecules of oxygen would react with 175 grams of propane? 175 g Molecules? ? ? C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g)

Stoichiometry Sequence 175 g ? ? ? L C 3 H 8 (g) + 175 grams C 3 H 8 5 O 2 (g) 1 mol C 3 H 8 44. 11 g C 3 H 8 5 3 CO 2 (g) + mol O 2 1 mol C 3 H 8 4 H 2 O (g) 6. 02 x 1023 cules O 2 1 mol O 2

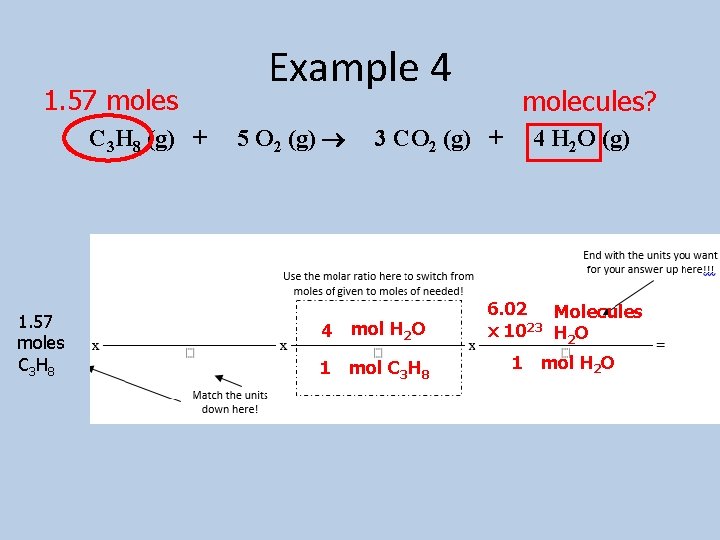
1. 57 moles C 3 H 8 (g) + 1. 57 moles C 3 H 8 Example 4 5 O 2 (g) 4 molecules? 3 CO 2 (g) + mol H 2 O 1 mol C 3 H 8 4 H 2 O (g) 6. 02 Molecules x 1023 H 2 O 1 mol H 2 O

N 2 + 3 H 2 2 NH 3 a) If 2. 5 moles of N 2 react, how many moles of NH 3 can be produced? b) If 10. 0 moles of NH 3 are produced, how many grams of H 2 would be needed? c) If 3. 50 grams of H 2 react, how many grams of NH 3 would be made? d) If 0. 60 grams of NH 3 are produced, how many molecules of N 2 are required?