Introduction to Statistical Thermodynamics Molecular Simulations u Molecular

- Slides: 35

Introduction to (Statistical) Thermodynamics

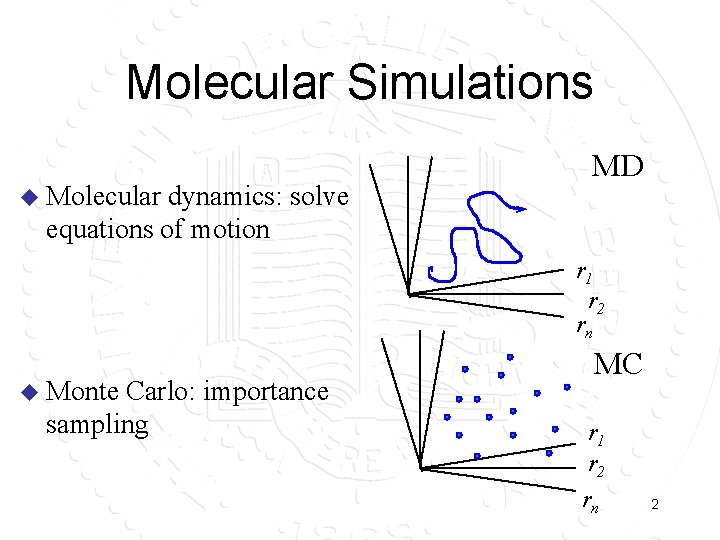
Molecular Simulations u Molecular dynamics: solve equations of motion MD r 1 r 2 rn u Monte Carlo: importance sampling MC r 1 r 2 rn 2

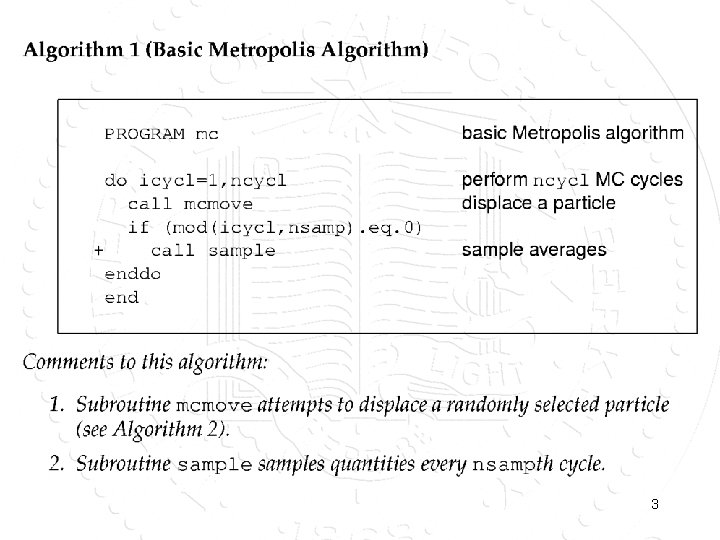
3

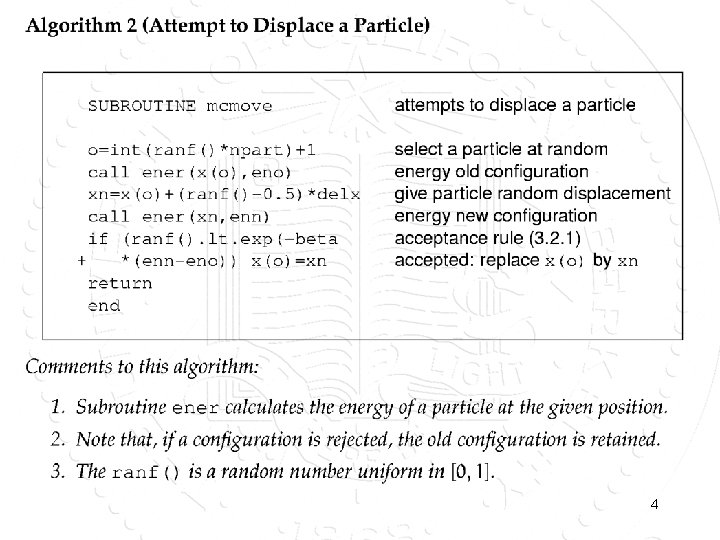
4

Questions. What is the desired distribution? • How can we prove that this scheme generates the desired distribution of configurations? • Why make a random selection of the particle to be displaced? • Why do we need to take the old configuration again? • How large should we take: delx? 5

Summary Thermodynamics – First law: conservation of energy – Second law: in a closed system entropy increase and takes its maximum value at equilibrium System at constant temperature and volume – Helmholtz free energy decrease and takes its minimum value at equilibrium Equilibrium: – Equal temperature, pressure, and chemical potential 6

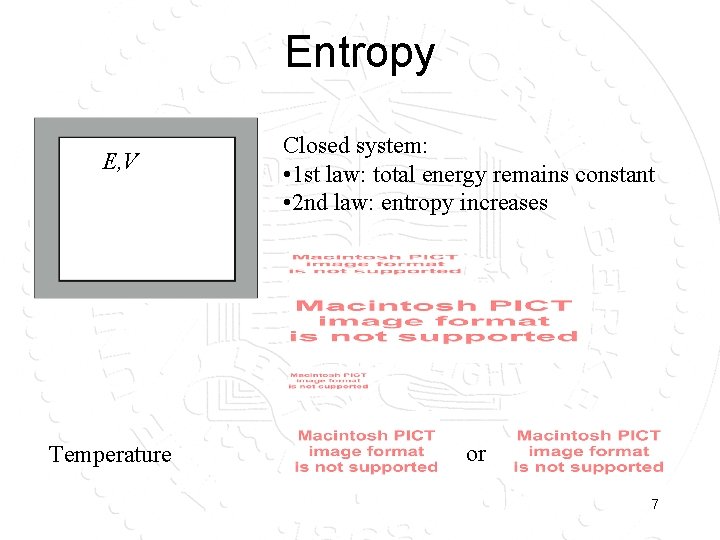
Entropy E, V Temperature Closed system: • 1 st law: total energy remains constant • 2 nd law: entropy increases or 7

Helmholtz free energy Pressure: Energy: Chemical potential: 8

Statistical Thermodynamics System of N molecules: But how do we obtain the macroscopic properties of this system from this microscopic information? 9

Outline (2) Statistical Thermodynamics – Basic Assumption • micro-canonical ensemble • relation to thermodynamics – Canonical ensemble • free energy • thermodynamic properties – Other ensembles • constant pressure • grand-canonical ensemble 10

Statistical Thermodynamics: the basics • • Nature is quantum-mechanical Consequence: – – • • Systems have discrete quantum states. For finite “closed” systems, the number of states is finite (but usually very large) Hypothesis: In a closed system, every state is equally likely to be observed. Consequence: ALL of equilibrium Statistical Mechanics and Thermodynamics 11

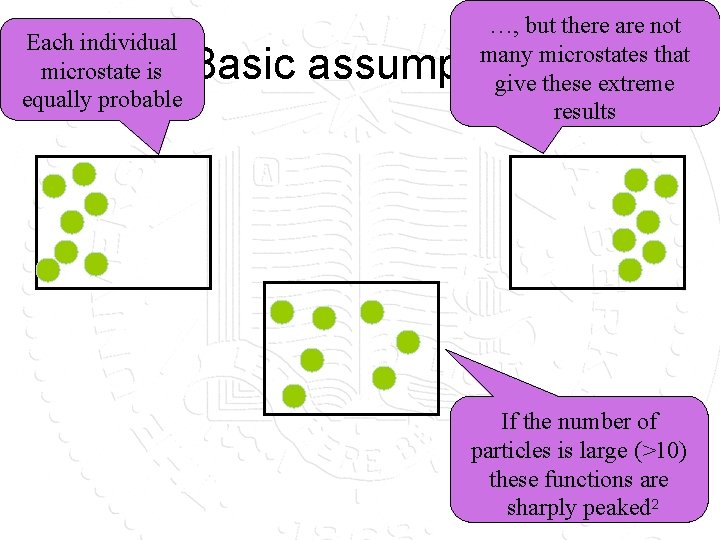
Each individual microstate is equally probable …, but there are not many microstates that give these extreme results Basic assumption If the number of particles is large (>10) these functions are sharply peaked 12

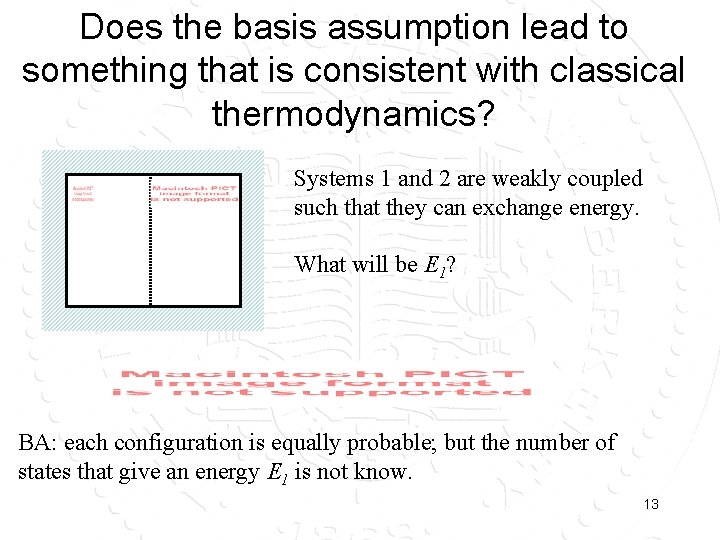
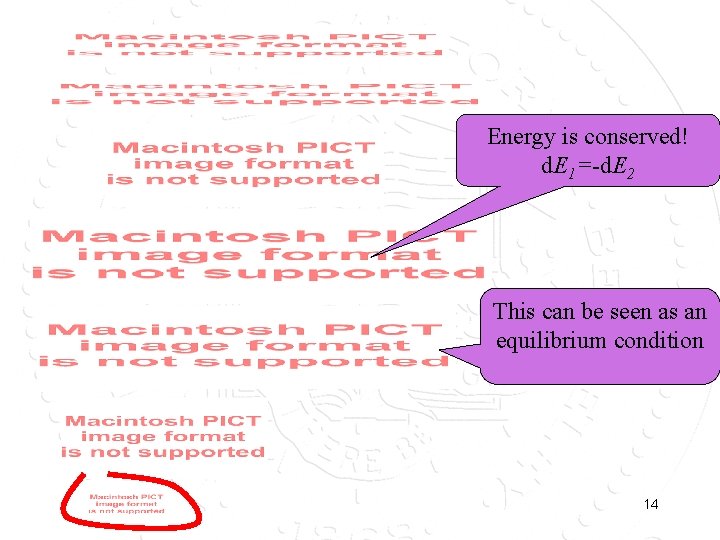
Does the basis assumption lead to something that is consistent with classical thermodynamics? Systems 1 and 2 are weakly coupled such that they can exchange energy. What will be E 1? BA: each configuration is equally probable; but the number of states that give an energy E 1 is not know. 13

Energy is conserved! d. E 1=-d. E 2 This can be seen as an equilibrium condition 14

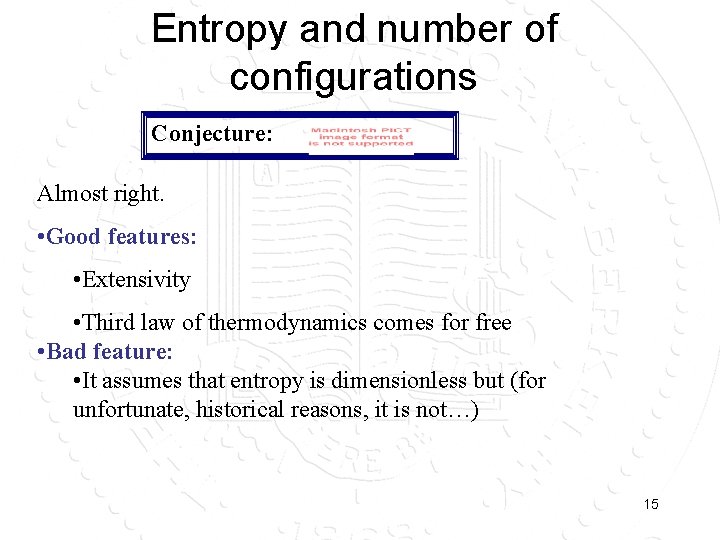
Entropy and number of configurations Conjecture: Almost right. • Good features: • Extensivity • Third law of thermodynamics comes for free • Bad feature: • It assumes that entropy is dimensionless but (for unfortunate, historical reasons, it is not…) 15

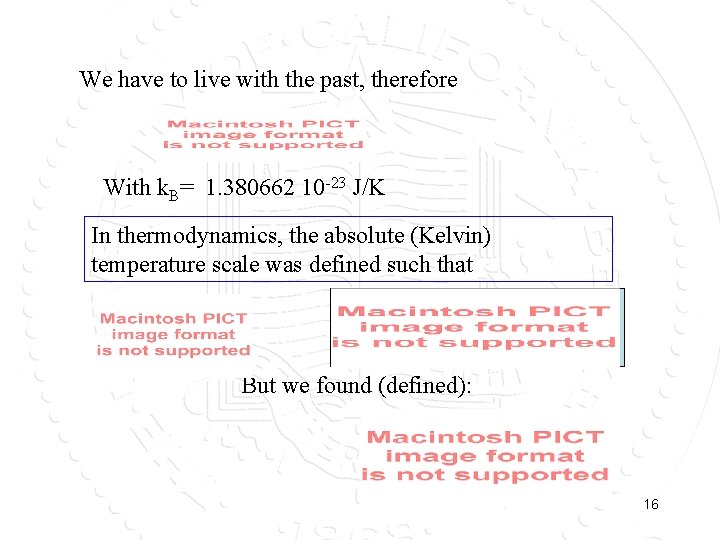
We have to live with the past, therefore With k. B= 1. 380662 10 -23 J/K In thermodynamics, the absolute (Kelvin) temperature scale was defined such that But we found (defined): 16

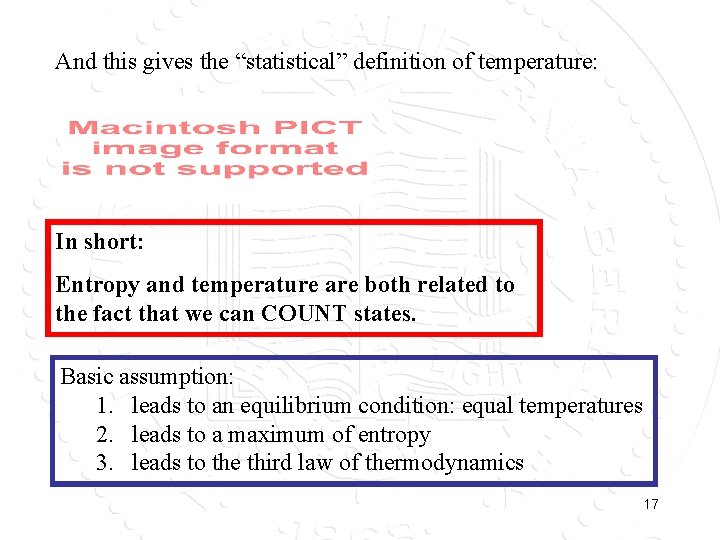
And this gives the “statistical” definition of temperature: In short: Entropy and temperature are both related to the fact that we can COUNT states. Basic assumption: 1. leads to an equilibrium condition: equal temperatures 2. leads to a maximum of entropy 3. leads to the third law of thermodynamics 17

Number of configurations How large is ? • For macroscopic systems, super-astronomically large. • For instance, for a glass of water at room temperature: • Macroscopic deviations from the second law of thermodynamics are not forbidden, but they are extremely unlikely. 18

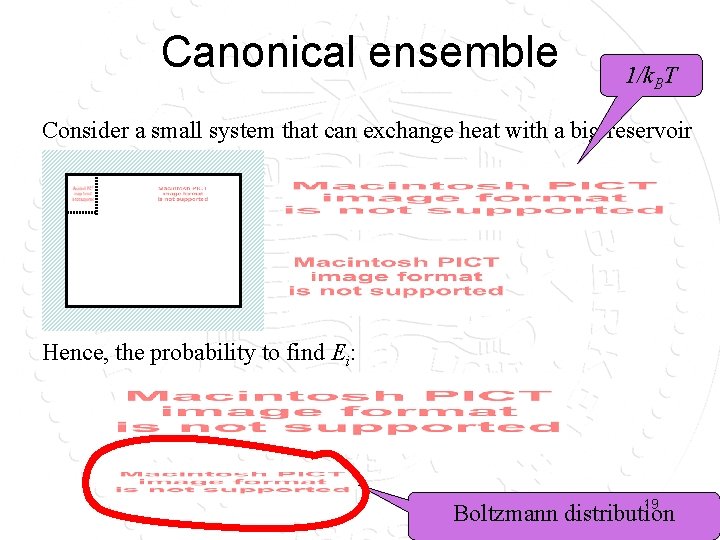
Canonical ensemble 1/k. BT Consider a small system that can exchange heat with a big reservoir Hence, the probability to find Ei: 19 Boltzmann distribution

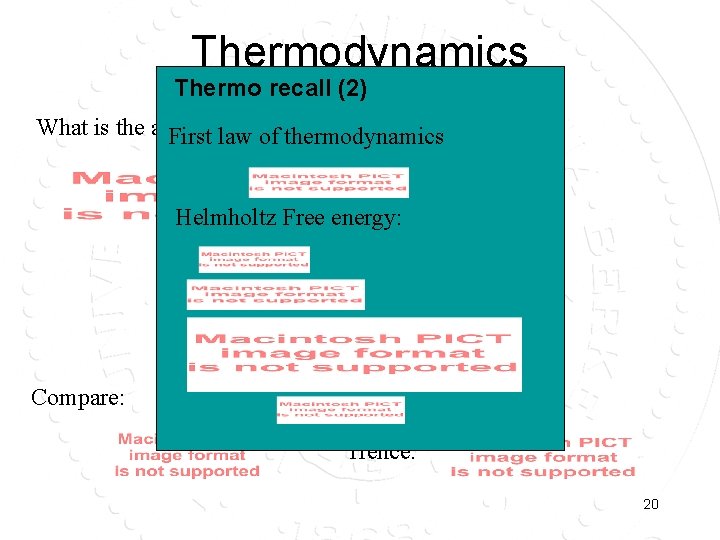
Thermodynamics Thermo recall (2) What is the average energy of the system? First law of thermodynamics Helmholtz Free energy: Compare: Hence: 20

Remarks (1) We have assume quantum mechanics (discrete states) but we are interested in the classical limit Volume of phase space (particle in a box) Particles are indistinguishable Integration over the momenta can be carried out for most systems: 21

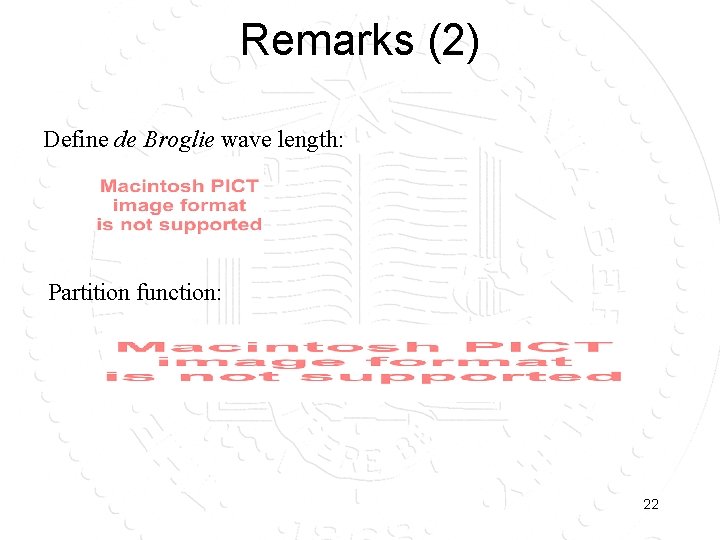
Remarks (2) Define de Broglie wave length: Partition function: 22

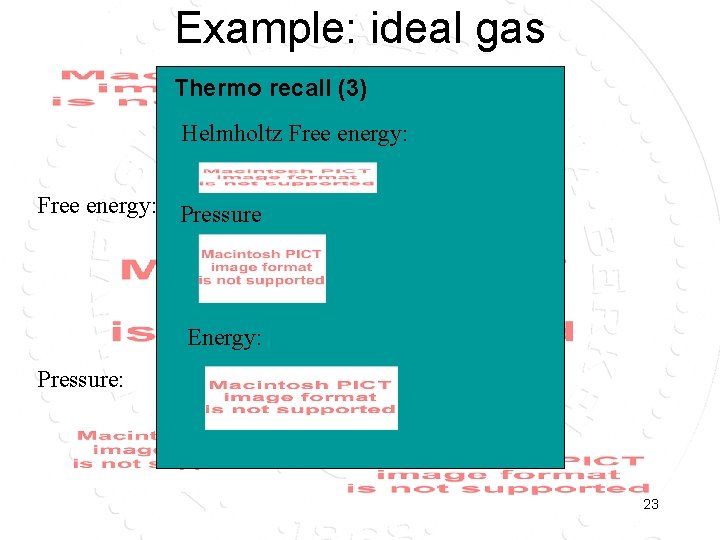
Example: ideal gas Thermo recall (3) Helmholtz Free energy: Pressure Energy: Pressure: Energy: 23

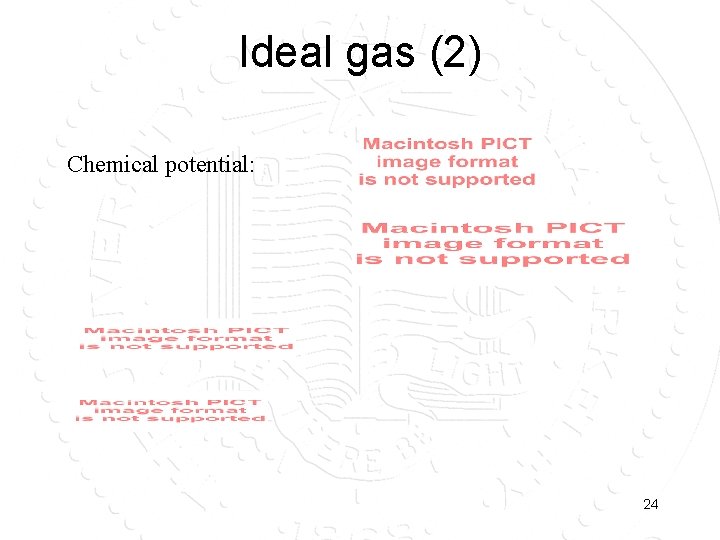
Ideal gas (2) Chemical potential: 24

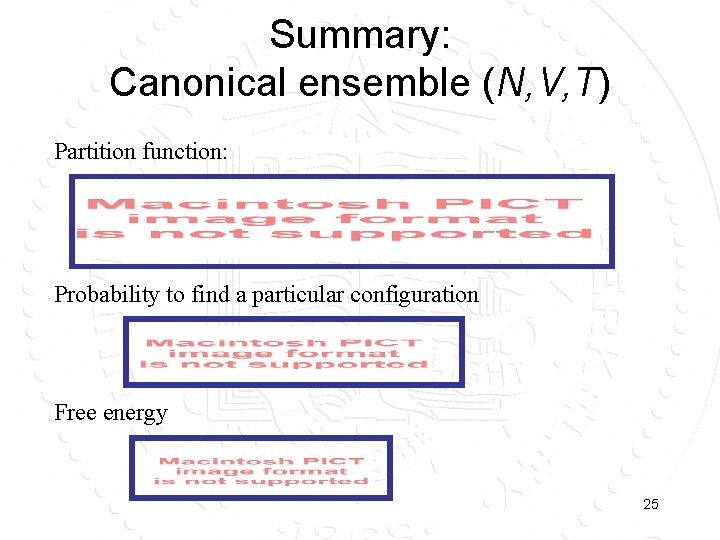
Summary: Canonical ensemble (N, V, T) Partition function: Probability to find a particular configuration Free energy 25

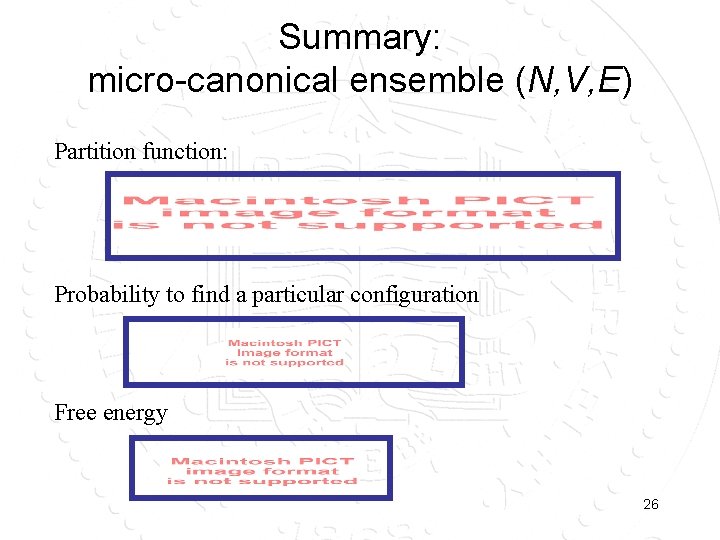
Summary: micro-canonical ensemble (N, V, E) Partition function: Probability to find a particular configuration Free energy 26

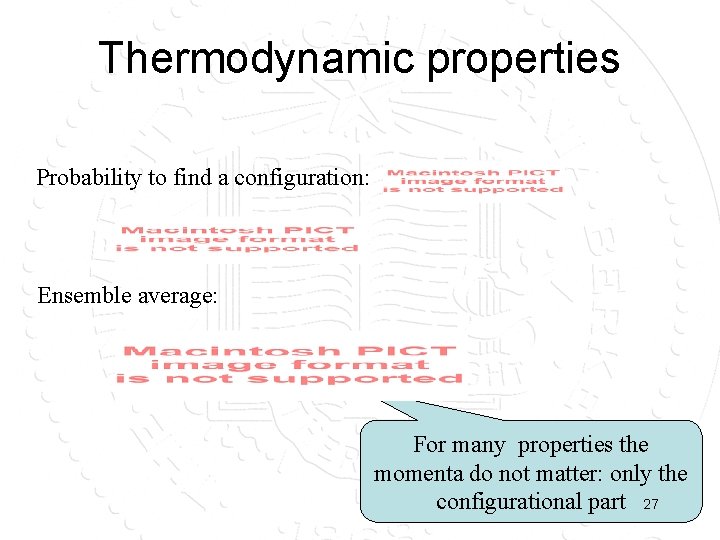
Thermodynamic properties Probability to find a configuration: Ensemble average: For many properties the momenta do not matter: only the configurational part 27

Other ensembles? COURSE: In thermodynamic limit thermodynamic are MD andproperties MC independent of the ensemble: so buy different a bigger computer ensembles… However, it is most of the times much better to think and to carefully select an appropriate ensemble. For this it is important to know how to simulate in the various ensembles. But for doing this wee need to know the Statistical Thermodynamics of the various ensembles. 28

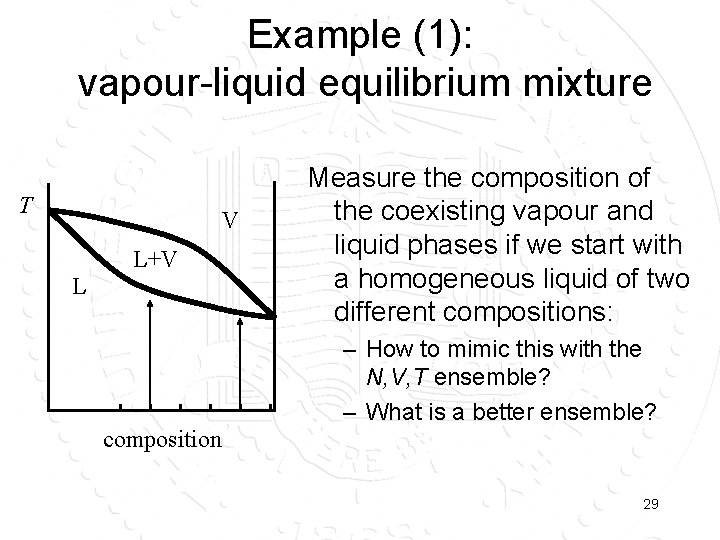
Example (1): vapour-liquid equilibrium mixture T V L+V L Measure the composition of the coexisting vapour and liquid phases if we start with a homogeneous liquid of two different compositions: – How to mimic this with the N, V, T ensemble? – What is a better ensemble? composition 29

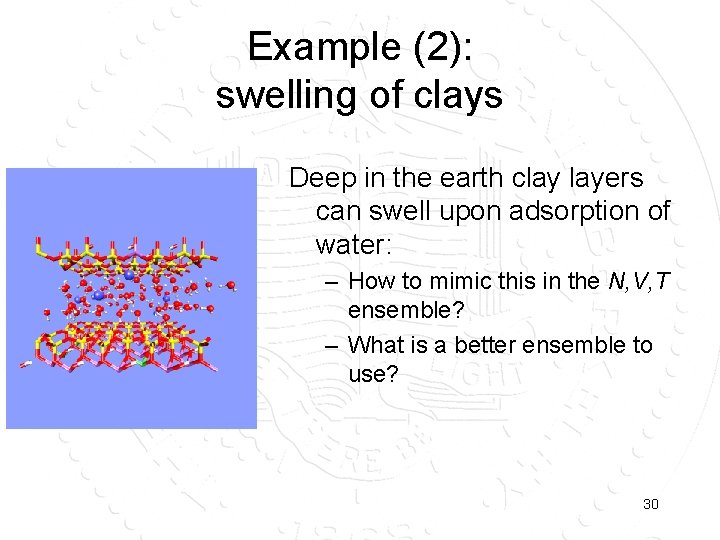
Example (2): swelling of clays Deep in the earth clay layers can swell upon adsorption of water: – How to mimic this in the N, V, T ensemble? – What is a better ensemble to use? 30

Ensembles • • Micro-canonical ensemble: E, V, N Canonical ensemble: T, V, N Constant pressure ensemble: T, P, N Grand-canonical ensemble: T, V, μ 31

Constant pressure simulations: N, P, T ensemble Thermo recall (4) 1/k. BT p/k. BT First law of. Consider thermodynamics a small system that can exchange volume and energy with a big reservoir Hence and Hence, the probability to find Ei, Vi: 32

N, P, T ensemble (2) In the classical limit, the partition function becomes The probability to find a particular configuration: 33

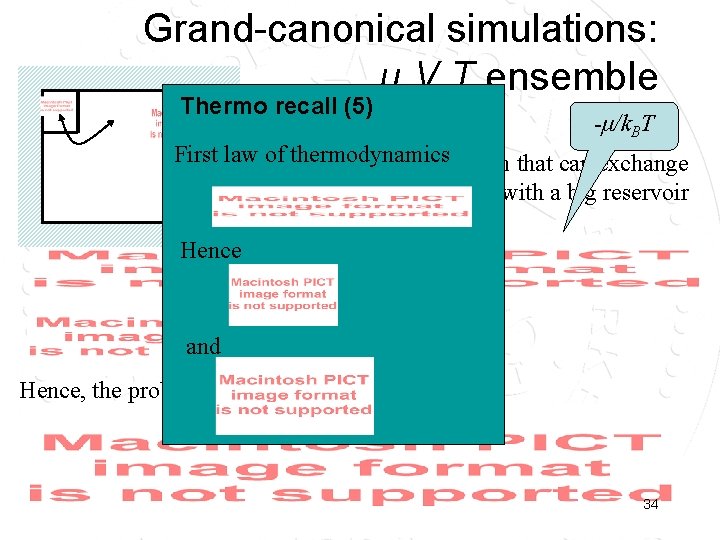
Grand-canonical simulations: μ, V, T ensemble Thermo recall (5) 1/k. BT -μ/k. BT First law of. Consider thermodynamics a small system that can exchange particles and energy with a big reservoir Hence and Hence, the probability to find Ei, Ni: 34

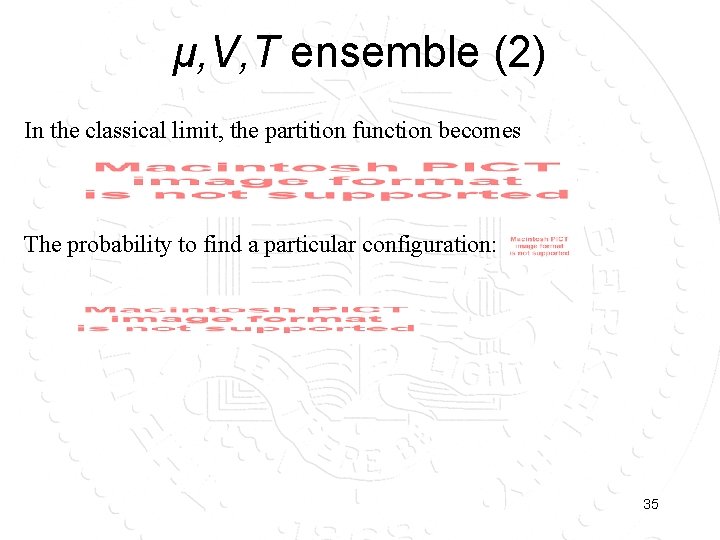
μ, V, T ensemble (2) In the classical limit, the partition function becomes The probability to find a particular configuration: 35