INTRODUCTION TO SPECTROPHOTOMETRY BACKGROUND l l Spectrophotometry is
INTRODUCTION TO SPECTROPHOTOMETRY

BACKGROUND l l Spectrophotometry is a method of analyzing that involves how light interacts with the atoms (or molecules) in a sample of matter. Visible light is only a small portion of the entire electromagnetic spectrum and it includes the colors commonly observed (red, yellow, green, blue and violet). The visible spectrum consists of electromagnetic radiation whose wavelengths range from 400 nm to nearly 800 nm.

BACKGROUND white light is observed, what is actually seen is a mixture of all the colors of light Why do some substances appear colored? When this light passes through a substance, certain energies (or colors) of the light are absorbed while other color(s) are allowed to pass through or are reflected by the substance. If the substance does not absorb any light, it appears white (all light is reflected) or colorless (all light is transmitted). A solution appears a certain color due to the absorbance and transmittance of visible light. For example, a blue solution appears blue because it is absorbing all of the colors except blue.

BACKGROUND A sample may also appear blue if all colors of light except yellow are transmitted. This is because blue and yellow are complementary colors. (See the color wheel above. )

BACKGROUND l l The amount of light absorbed by a solution is dependent on the ability of the compound to absorb light (molar absorptivity), the distance through which the light must pass through the sample (path length) and the molar concentration of the compound in the solution. If the same compound is being used and the path length is kept constant, then the absorbance is directly proportional to the concentration of the sample.
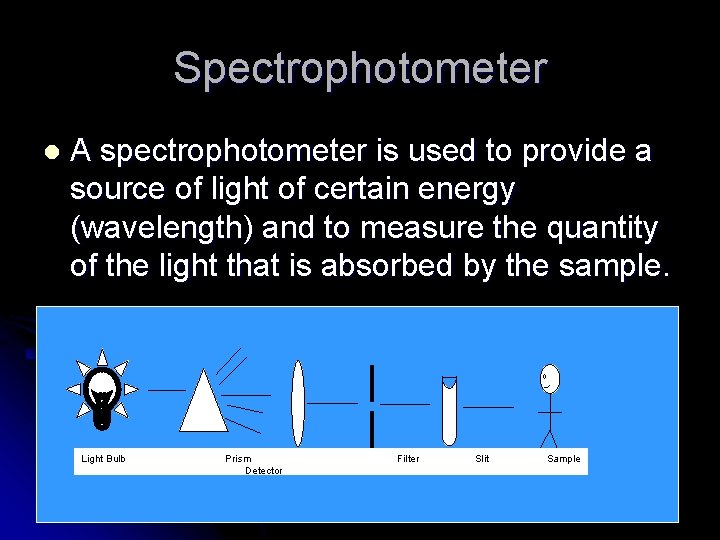
Spectrophotometer l A spectrophotometer is used to provide a source of light of certain energy (wavelength) and to measure the quantity of the light that is absorbed by the sample. Light Bulb Prism Detector Filter Slit Sample
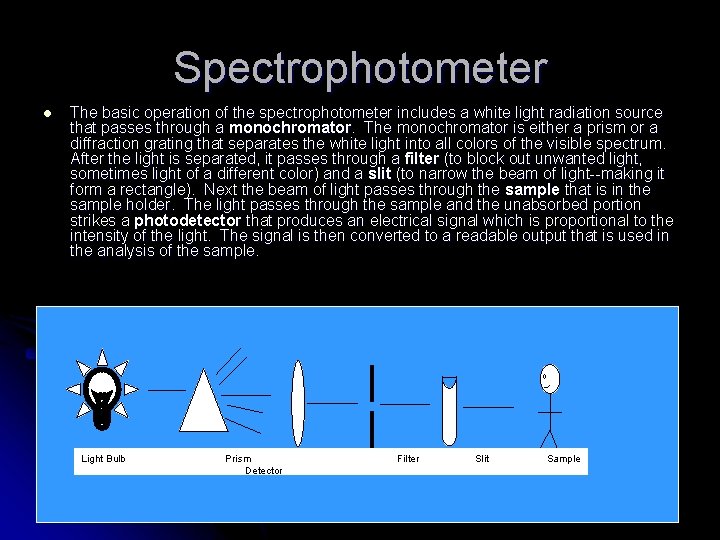
Spectrophotometer l The basic operation of the spectrophotometer includes a white light radiation source that passes through a monochromator. The monochromator is either a prism or a diffraction grating that separates the white light into all colors of the visible spectrum. After the light is separated, it passes through a filter (to block out unwanted light, sometimes light of a different color) and a slit (to narrow the beam of light--making it form a rectangle). Next the beam of light passes through the sample that is in the sample holder. The light passes through the sample and the unabsorbed portion strikes a photodetector that produces an electrical signal which is proportional to the intensity of the light. The signal is then converted to a readable output that is used in the analysis of the sample. Light Bulb Prism Detector Filter Slit Sample
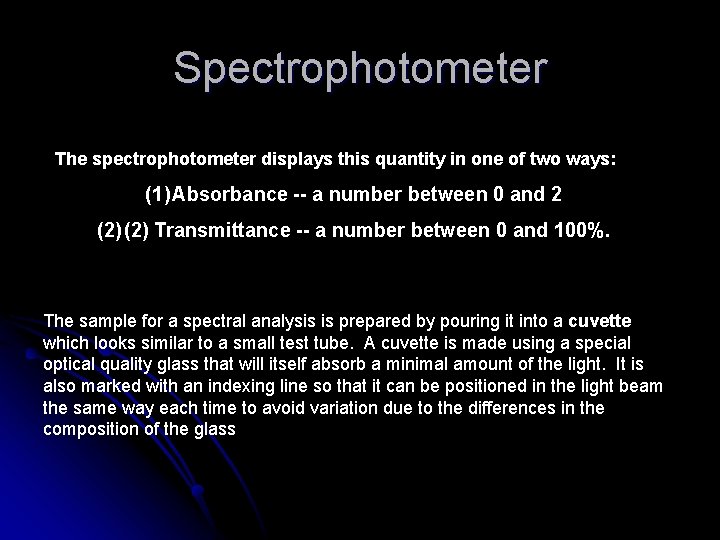
Spectrophotometer The spectrophotometer displays this quantity in one of two ways: (1) Absorbance -- a number between 0 and 2 (2) Transmittance -- a number between 0 and 100%. The sample for a spectral analysis is prepared by pouring it into a cuvette which looks similar to a small test tube. A cuvette is made using a special optical quality glass that will itself absorb a minimal amount of the light. It is also marked with an indexing line so that it can be positioned in the light beam the same way each time to avoid variation due to the differences in the composition of the glass
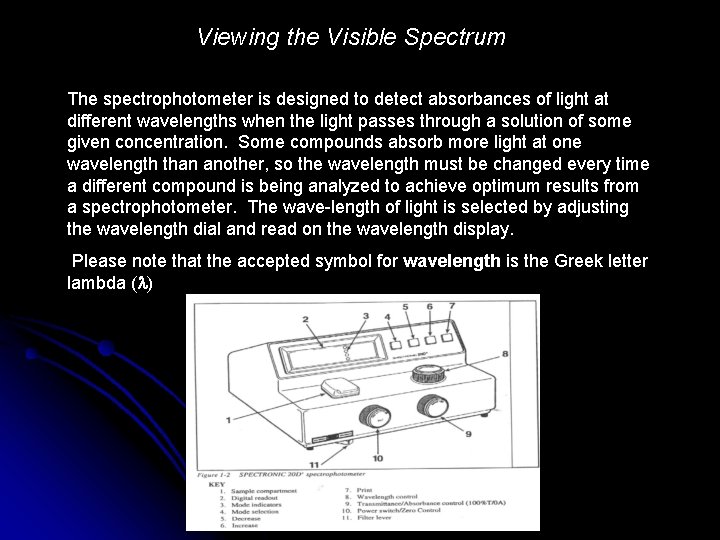
Viewing the Visible Spectrum The spectrophotometer is designed to detect absorbances of light at different wavelengths when the light passes through a solution of some given concentration. Some compounds absorb more light at one wavelength than another, so the wavelength must be changed every time a different compound is being analyzed to achieve optimum results from a spectrophotometer. The wave-length of light is selected by adjusting the wavelength dial and read on the wavelength display. Please note that the accepted symbol for wavelength is the Greek letter lambda ( )

A visible spectrophotometer can be used to learn why colored solutions appear a particular color. For example, WHY does blue food coloring appear blue? Simply put, the solution is blue because it transmits (and reflects) blue visible light more than it transmits other colors of visible light. In other words, blue food coloring absorbs blue visible light the least and absorbs other colors of light more. When white light is observed, what is actually being seen is a mixture of all the colors of light. When this light passes through a substance, certain energies (or colors) the light are absorbed while other color(s) are allowed to pass through or are reflected by the substance. This is why some substances appear colored. The color that we see is the combination of energies of visible light which are not absorbed by the sample. If the substance does not absorb any light, it appears white or colorless.

Two ways to make colors A solution appears a certain color due to the absorbance and transmittance of visible light. For example, the blue solution appears blue because it is absorbing all of the colors except blue. A sample may also appear blue if all colors of light except yellow are transmitted (yellow is absorbed). This is because blue and yellow are complementary colors. Any two colors opposite each other on the color wheel (see figure above) are said to be complementary. The wavelength (numbers outside the wheel) associated with the complementary color is known as the wavelength of maximum absorbance. This is because in a colored solution the maximum amount of light is absorbed by the complementary color. Note: cyan = green.

Basic Principles of Gas Chromatography
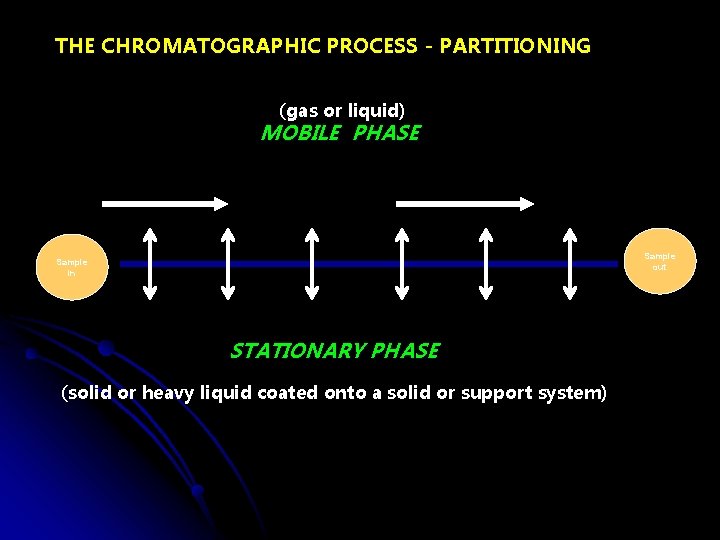
THE CHROMATOGRAPHIC PROCESS - PARTITIONING (gas or liquid) MOBILE PHASE Sample out Sample in STATIONARY PHASE (solid or heavy liquid coated onto a solid or support system)
Columns • Packed • Capillary
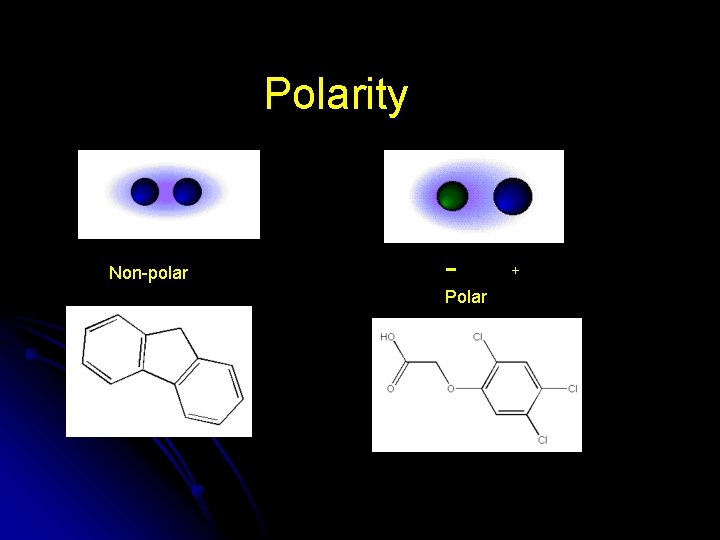
Polarity Non-polar Polar +
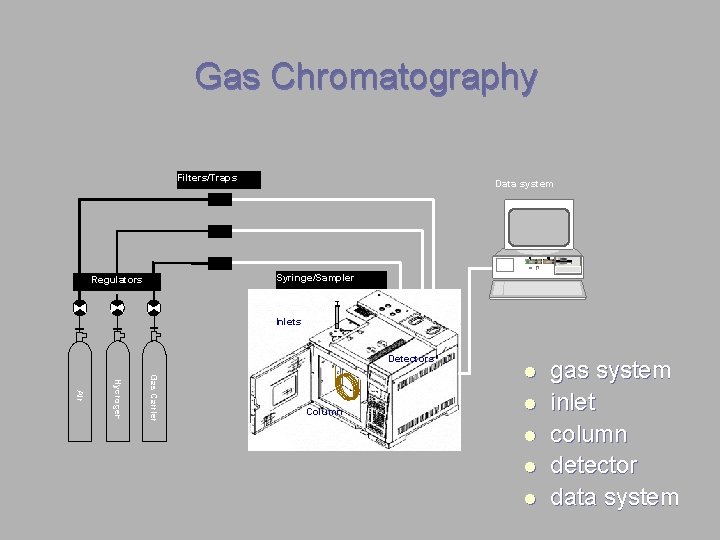
Gas Chromatography Filters/Traps Data system H RESET Syringe/Sampler Regulators Inlets Detectors Gas Carrier Hydrogen Air Column l l l gas system inlet column detector data system
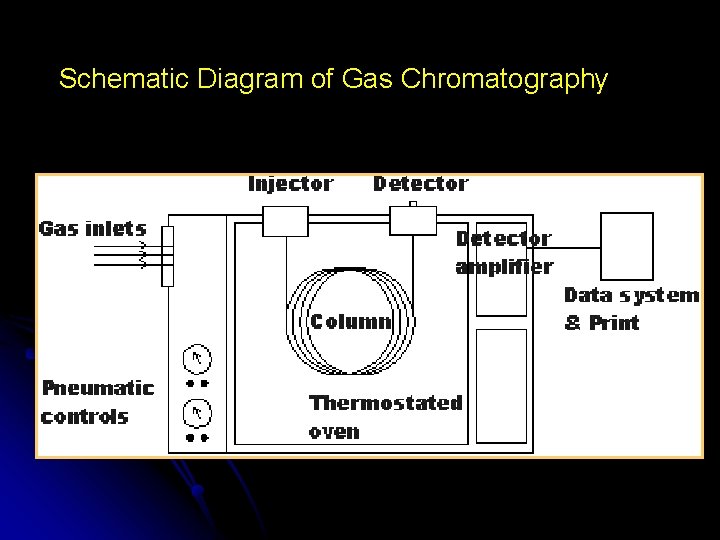
Schematic Diagram of Gas Chromatography
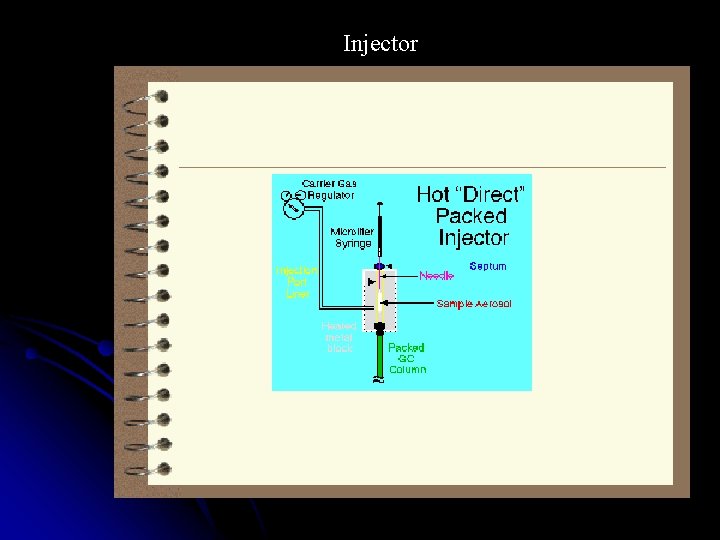
Injector
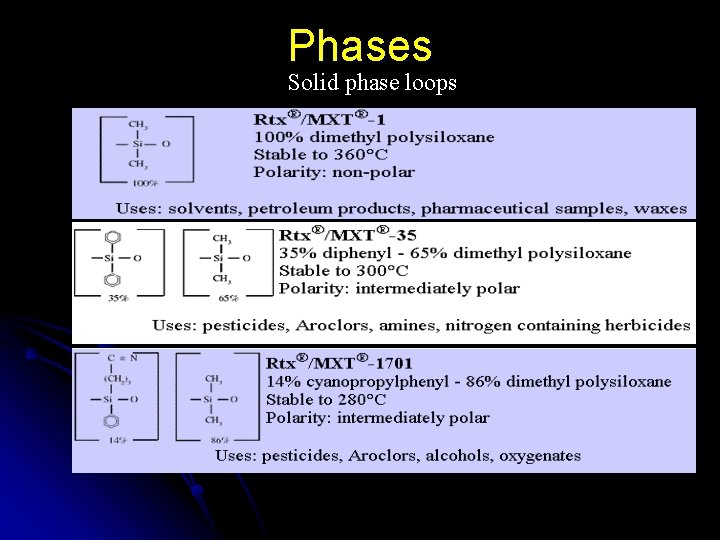
Phases Solid phase loops
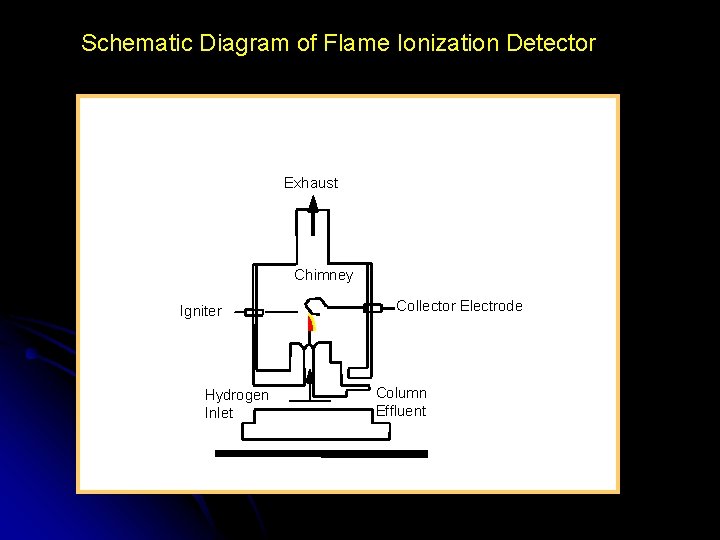
Schematic Diagram of Flame Ionization Detector Exhaust Chimney Igniter Hydrogen Inlet Collector Electrode Column Effluent
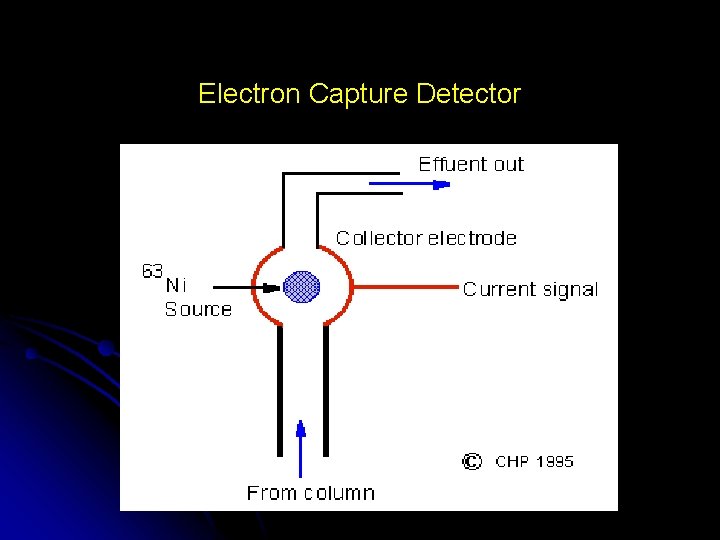
Electron Capture Detector
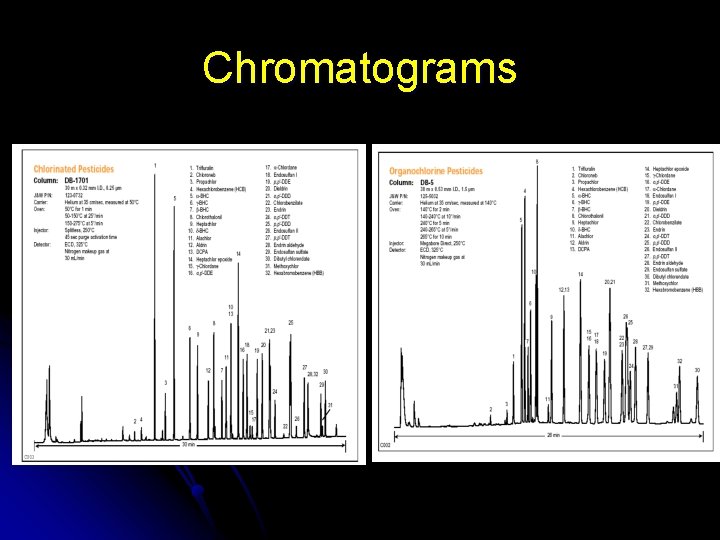
Chromatograms
- Slides: 22