Introduction to Special Coagulation Ahmad Sh Silmi Hematology




















- Slides: 70

Introduction to Special Coagulation Ahmad Sh. Silmi Hematology Lecturer Medical Tech. Dept IUG 1

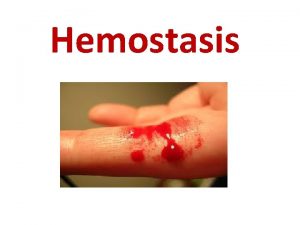
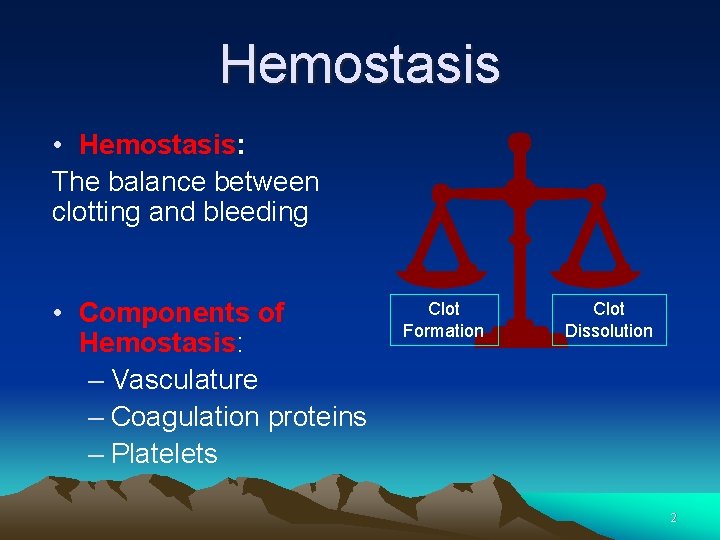
Hemostasis • Hemostasis: The balance between clotting and bleeding • Components of Hemostasis: – Vasculature – Coagulation proteins – Platelets Clot Formation Clot Dissolution 2

Blood Vessels ü Intact endothelium forms a thromboresistant surface ü endothelial cells have additional synthetic functions. 3

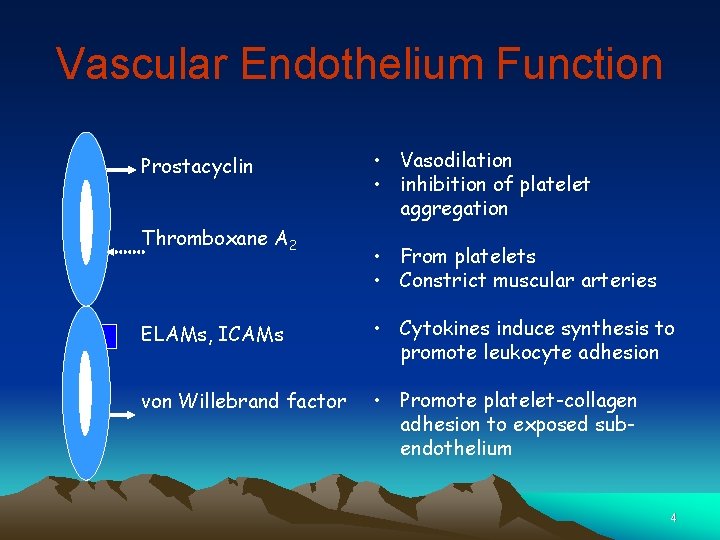
Vascular Endothelium Function Prostacyclin Thromboxane A 2 • Vasodilation • inhibition of platelet aggregation • From platelets • Constrict muscular arteries ELAMs, ICAMs • Cytokines induce synthesis to promote leukocyte adhesion von Willebrand factor • Promote platelet-collagen adhesion to exposed subendothelium 4

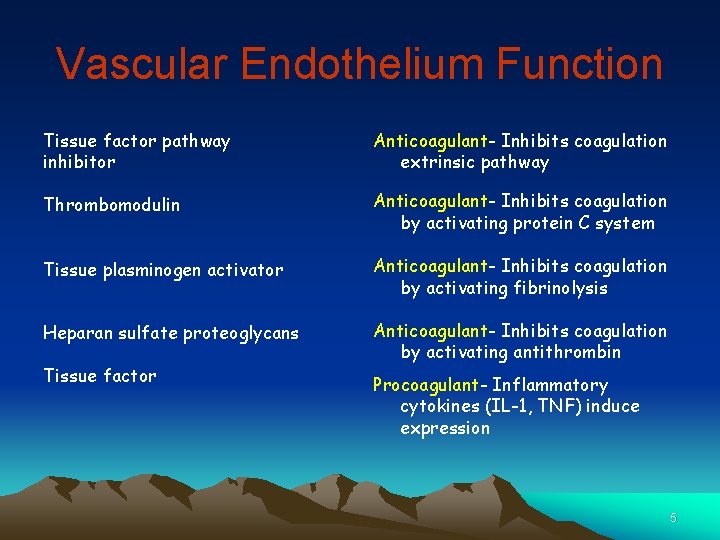
Vascular Endothelium Function Tissue factor pathway inhibitor Anticoagulant- Inhibits coagulation extrinsic pathway Thrombomodulin Anticoagulant- Inhibits coagulation by activating protein C system Tissue plasminogen activator Anticoagulant- Inhibits coagulation by activating fibrinolysis Heparan sulfate proteoglycans Anticoagulant- Inhibits coagulation by activating antithrombin Tissue factor Procoagulant- Inflammatory cytokines (IL-1, TNF) induce expression 5

Blood Platelets are formed from the cytoplasm of bone marrow megakaryocytes and are the smallest of the blood cells. • Normal platelet count lies between 150 -400 x 109/L • Disc-shaped, anucleated cells with complex internal structure reflecting the specific hemostatic functions of the platelet. 6

Blood Platelets (cont’d) • Two major types of intracellular granules: • --α-granules contain: – coagulation factors (fibrinogen, von Willebrand Factor, and coagulation factors V and VIII) – platelet-derived growth factor (PDGF). • --dense granules (because of their appearance on EM) contain: – ADP, ATP and serotonin. • α- and dense granules contents released by platelet activation. 7

Diagrammatic Representation of the Platelet 8

PLATELET ACTIVATION PATHWAYS 9

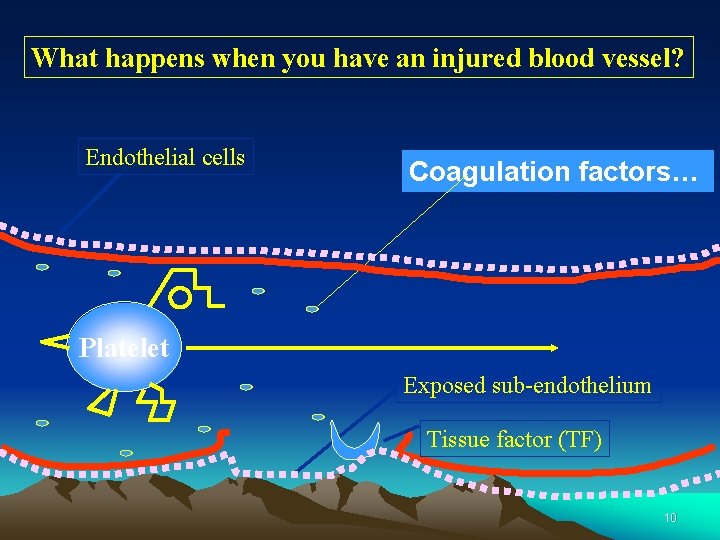
What happens when you have an injured blood vessel? Endothelial cells Coagulation factors… Platelet Exposed sub-endothelium Tissue factor (TF) 10

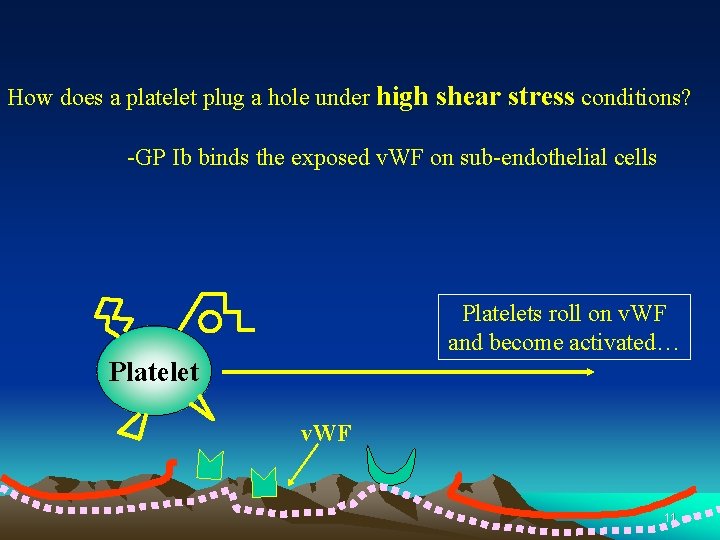
How does a platelet plug a hole under high shear stress conditions? -GP Ib binds the exposed v. WF on sub-endothelial cells Platelets roll on v. WF and become activated… Platelet v. WF 11

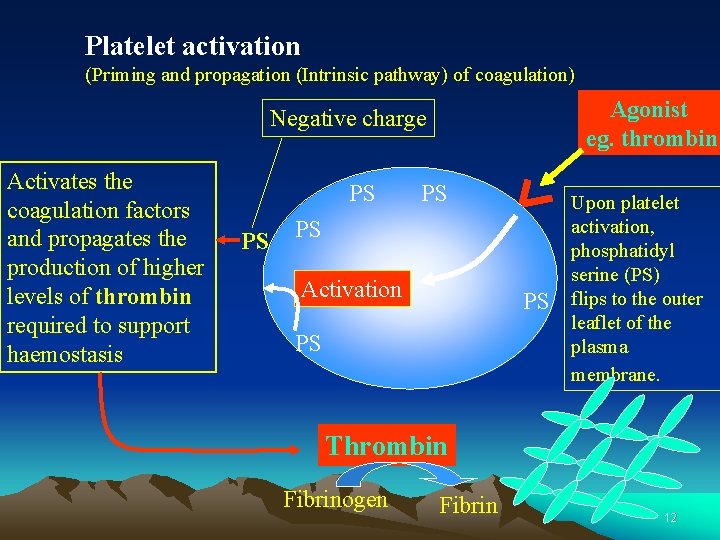
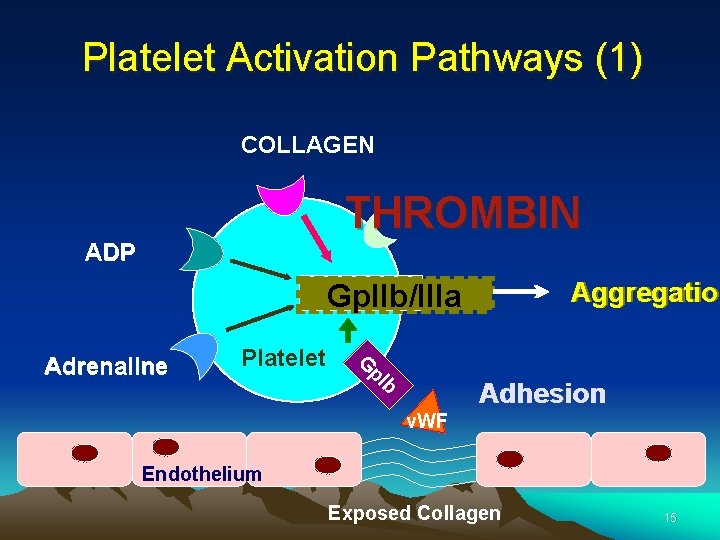
Platelet activation (Priming and propagation (Intrinsic pathway) of coagulation) Agonist eg. thrombin Negative charge Activates the coagulation factors and propagates the production of higher levels of thrombin required to support haemostasis PS PS Activation PS PS Upon platelet activation, phosphatidyl serine (PS) flips to the outer leaflet of the plasma membrane. Thrombin Fibrinogen Fibrin 12

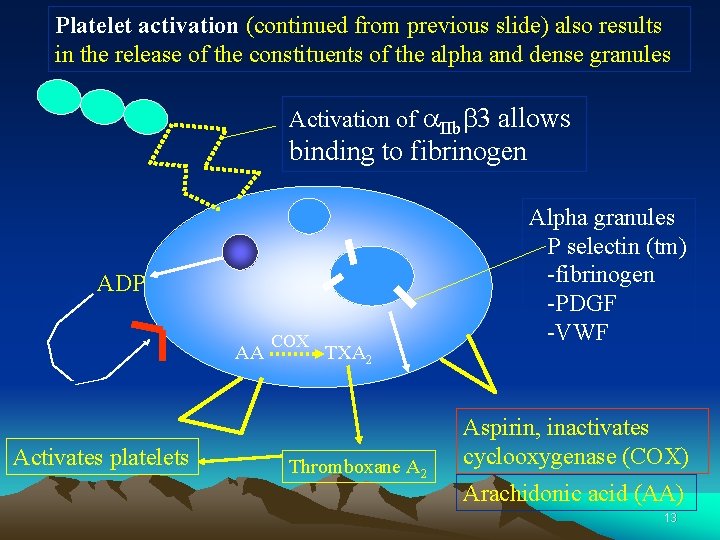
Platelet activation (continued from previous slide) also results in the release of the constituents of the alpha and dense granules Activation of a. IIbb 3 allows binding to fibrinogen ADP AA Activates platelets COX TXA 2 Thromboxane A 2 Alpha granules P selectin (tm) -fibrinogen -PDGF -VWF Aspirin, inactivates cyclooxygenase (COX) Arachidonic acid (AA) 13

Platelet activation (cont. ): - a. IIbb 3 becomes activated and capable of binding fibrinogen Platelet Fibrinogen The co-crosslinking of two or more platelets by a. IIbb 3 and fibrinogen is termed ‘aggregation’ 14

Platelet Activation Pathways (1) COLLAGEN THROMBIN ADP Aggregation Aggregatio Gp. IIb/IIIa Platelet G p. I Adrenaline b Adhesion v. WF Endothelium Exposed Collagen 15

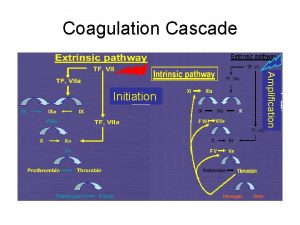
Coagulation Cascade 16

Coagulation Cascade – Then & Now • “Waterfall” theory developed in the 1960’s – Believed that clotting occurs through a series of reactions in which serine protease zymogens are converted into active enzymes in a step-wise process • For many years, intrinsic pathway was believed to be the more important clotting mechanism; didn’t take into account the significance of the extrinsic pathway (TF/FVII pathway) 17

Coagulation Cascade • Vascular damage initiates the coagulation cascade. • Results in the generation of thrombin at the site of injury. • Thrombin catalyzes the conversion of fibrinogen to an insoluble fibrin (clot) matrix. 18

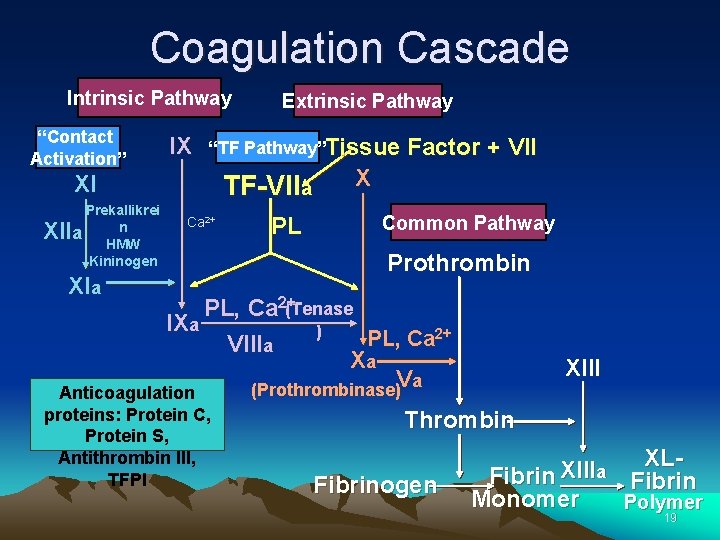
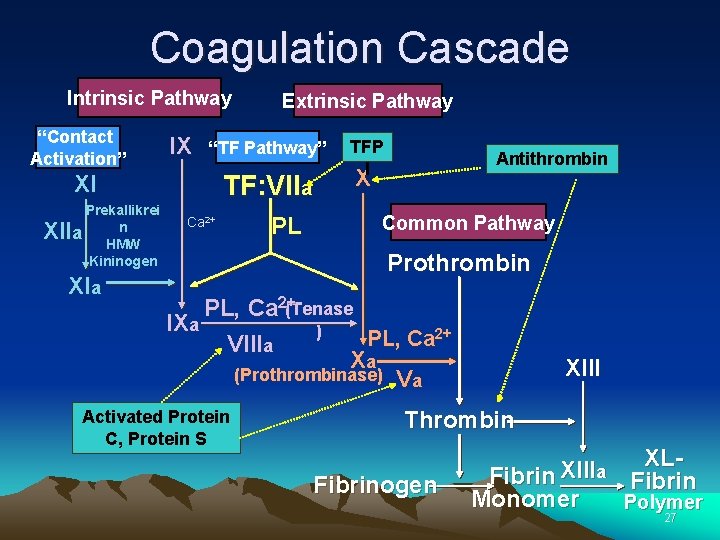
Coagulation Cascade Intrinsic Pathway “Contact Activation” IX “TF Pathway”Tissue XI Prekallikrei n a HMW Kininogen XII Extrinsic Pathway TF-VIIa Ca 2+ XIa PL Factor + VII X Common Pathway Prothrombin PL, Ca 2+(Tenase IXa ) PL, Ca 2+ VIIIa Xa Va (Prothrombinase) Anticoagulation proteins: Protein C, Protein S, Antithrombin III, TFPI XIII Thrombin Fibrinogen XLXIII a Fibrin Monomer Polymer 19

Coagulation Cascade • Abnormal activation of blood coagulation and/or depressed fibrinolytic activity may lead to the formation of a thrombus (clot). • In contrast, a defect or deficiency in the coagulation process and/or accelerated fibrinolysis is associated with a bleeding tendency. 20

Coagulation Cascade • The cascade scheme is organized into the INTRINSIC and EXTRINSIC pathways, converging into the COMMON pathway. 21

Intrinsic Pathway Contact Activation IX X XI Prekallikrei n a HMW Kininogen XII Ca 2+ XIa IXa Xa Intrinsic Pathway “Contact Activation”: Initiated by the activation of FXII involving contact factors on negatively-charged phospholipid surfaces (glass or kaolin in vitro) • Factors XII, XI, IX, VIII, prekallikrein, HMW kininogen • Measured with a. PTT clotting assay 22

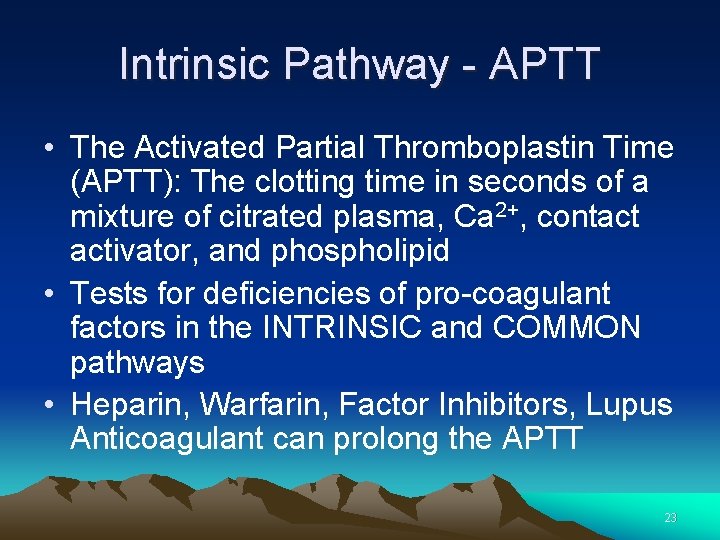
Intrinsic Pathway - APTT • The Activated Partial Thromboplastin Time (APTT): The clotting time in seconds of a mixture of citrated plasma, Ca 2+, contact activator, and phospholipid • Tests for deficiencies of pro-coagulant factors in the INTRINSIC and COMMON pathways • Heparin, Warfarin, Factor Inhibitors, Lupus Anticoagulant can prolong the APTT 23

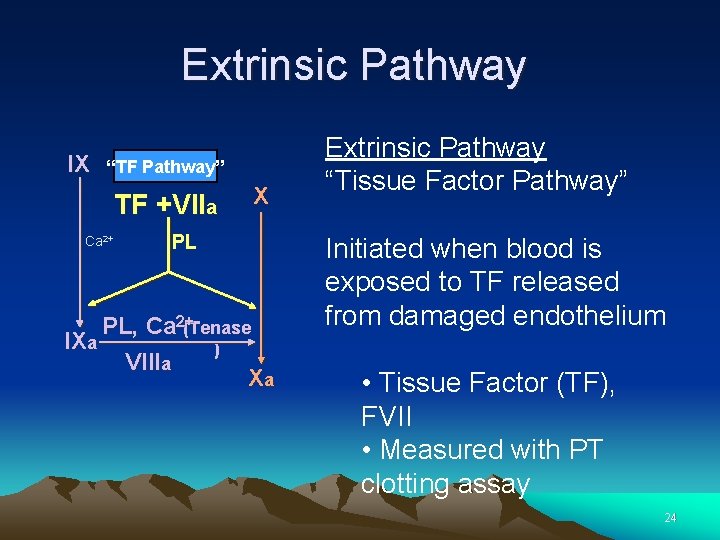
Extrinsic Pathway IX “TF Pathway” TF +VIIa Ca 2+ X PL PL, Ca 2+(Tenase IXa ) VIIIa Xa Extrinsic Pathway “Tissue Factor Pathway” Initiated when blood is exposed to TF released from damaged endothelium • Tissue Factor (TF), FVII • Measured with PT clotting assay 24

Extrinsic Pathway - PT • Prothrombin Time (PT): clotting time in seconds of a mixture of thromboplastin (Tissue Factor) reagent and citrated plasma in the presence of Ca 2+ • Tests for deficiencies of pro-coagulant factors of the EXTRINSIC and COMMON pathways 25

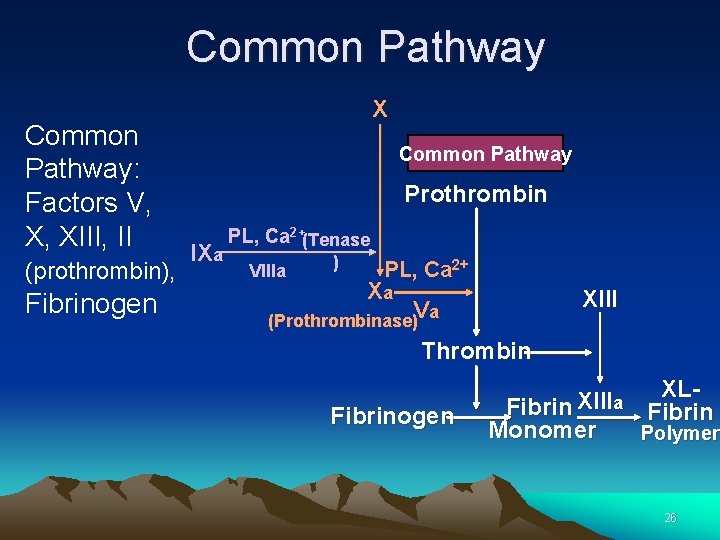
Common Pathway: Factors V, X, XIII, II (prothrombin), Fibrinogen X Common Pathway Prothrombin IXa PL, Ca 2+(Tenase VIIIa ) PL, Ca 2+ Xa XIII Va (Prothrombinase) Thrombin Fibrinogen XLXIII a Fibrin Monomer Polymer 26

Coagulation Cascade Intrinsic Pathway “Contact Activation” IX “TF Pathway” XI Prekallikrei n a HMW Kininogen XII XIa Extrinsic Pathway TF: VIIa Ca 2+ PL TFP I Antithrombin X Common Pathway Prothrombin PL, Ca 2+(Tenase IXa ) PL, Ca 2+ VIIIa Xa (Prothrombinase) Va Activated Protein C, Protein S XIII Thrombin Fibrinogen XLXIII a Fibrin Monomer Polymer 27

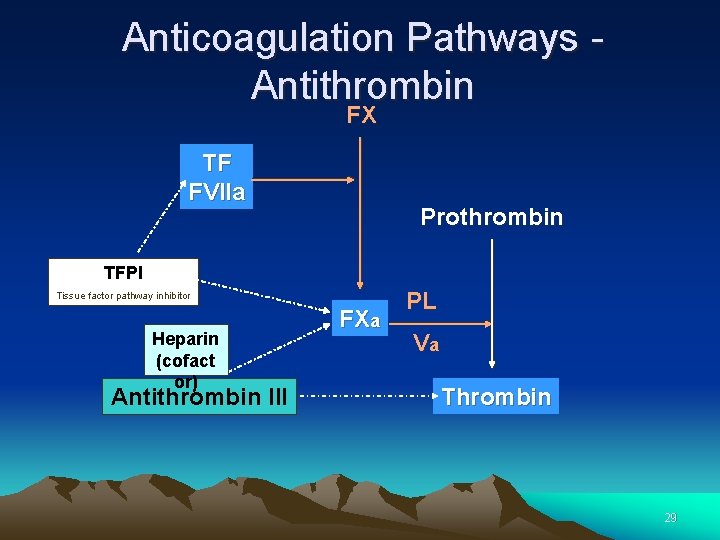
Anticoagulation Pathways 1. Antithrombin 2. Protein C thrombomodulin pathway 3. Tissue Factor Pathway Inhibitor (TFPI) Activity 28

Anticoagulation Pathways - Antithrombin FX TF FVIIa Prothrombin TFPI Tissue factor pathway inhibitor Heparin (cofact or) Antithrombin III FXa PL Va Thrombin 29

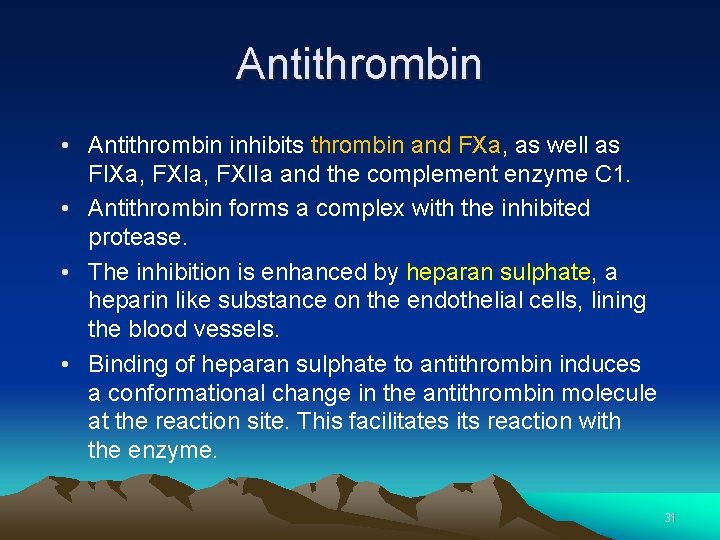
Anticoagulation Pathways Antithrombin • Antithrombin is the major inhibitor of thrombin, accounting for approximately 80% of thrombin inhibitory activity in plasma • Antithrombin primarily inhibits Thrombin and FXa 30

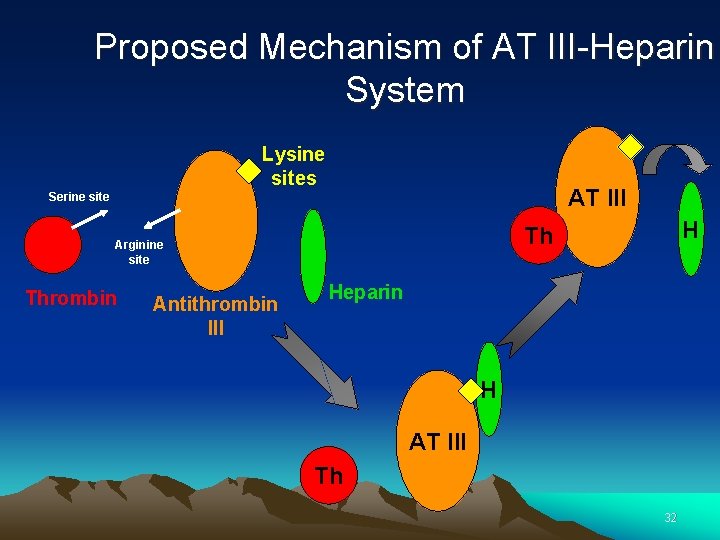
Antithrombin • Antithrombin inhibits thrombin and FXa, as well as FIXa, FXIIa and the complement enzyme C 1. • Antithrombin forms a complex with the inhibited protease. • The inhibition is enhanced by heparan sulphate, a heparin like substance on the endothelial cells, lining the blood vessels. • Binding of heparan sulphate to antithrombin induces a conformational change in the antithrombin molecule at the reaction site. This facilitates its reaction with the enzyme. 31

Proposed Mechanism of AT III-Heparin System Lysine sites AT III Serine site Thrombin Antithrombin III H Th Arginine site Heparin H AT III Th 32

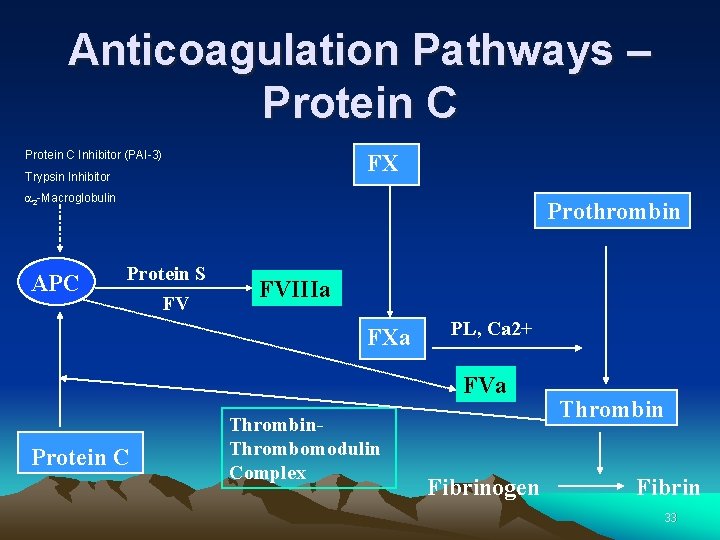
Anticoagulation Pathways – Protein C Inhibitor (PAI-3) FX Trypsin Inhibitor a 2 -Macroglobulin APC Prothrombin Protein S FV FVIIIa FXa PL, Ca 2+ FVa Protein C Thrombin. Thrombomodulin Complex Fibrinogen Thrombin Fibrin 33

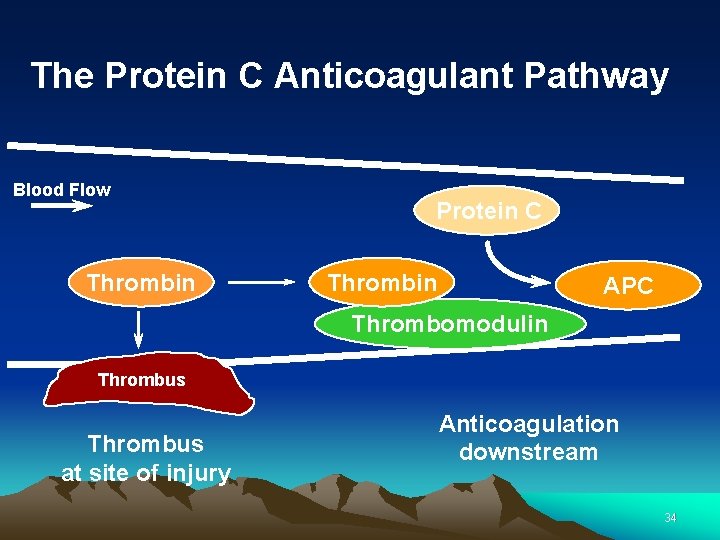
The Protein C Anticoagulant Pathway Blood Flow Thrombin Protein C Thrombin APC Thrombomodulin Thrombus at site of injury Anticoagulation downstream 34

The Protein C Anticoagulant Pathway Blood Flow Vai Factor V Leiden Va APC PS VIIIai VIIIa APC PS Thrombus 35

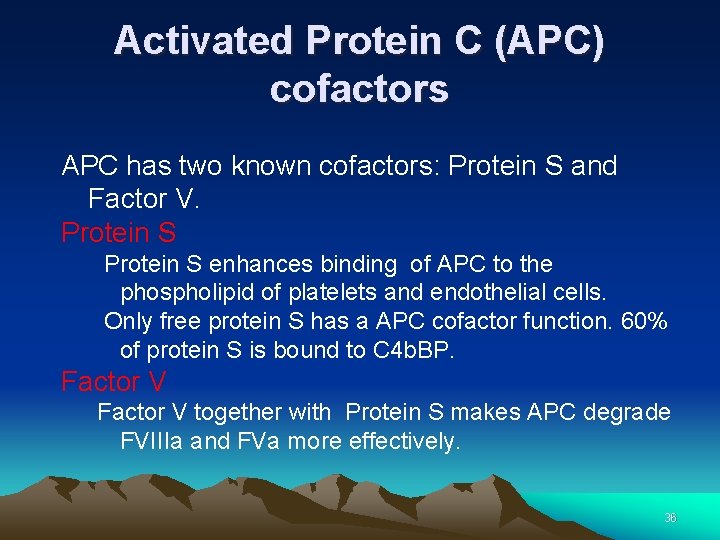
Activated Protein C (APC) cofactors APC has two known cofactors: Protein S and Factor V. Protein S enhances binding of APC to the phospholipid of platelets and endothelial cells. Only free protein S has a APC cofactor function. 60% of protein S is bound to C 4 b. BP. Factor V together with Protein S makes APC degrade FVIIIa and FVa more effectively. 36

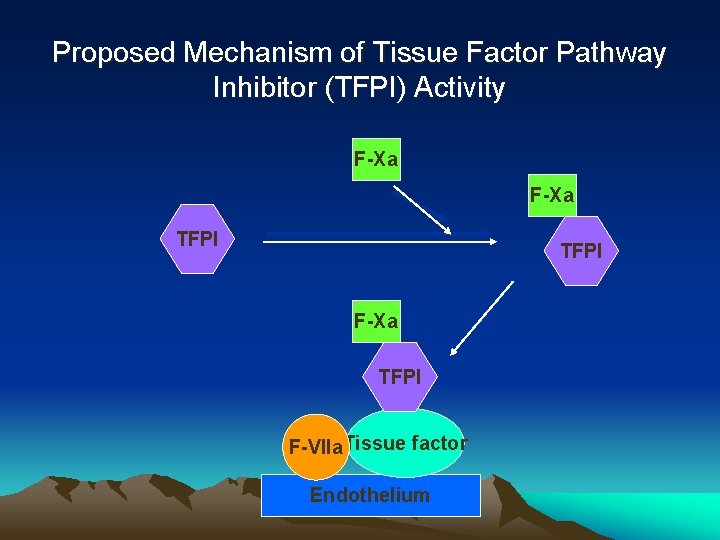
Proposed Mechanism of Tissue Factor Pathway Inhibitor (TFPI) Activity F-Xa TFPI F-VIIa. Tissue factor Endothelium

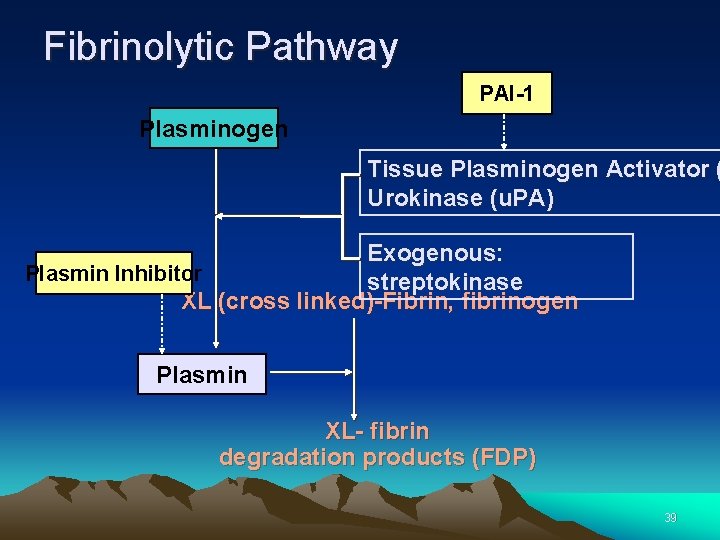
Fibrinolytic Pathway Fibrinolysis is initiated when fibrin is formed and eventually dissolves the clot. 38

Fibrinolytic Pathway PAI-1 Plasminogen Tissue Plasminogen Activator ( Urokinase (u. PA) Exogenous: Plasmin Inhibitor streptokinase XL (cross linked)-Fibrin, fibrinogen Plasmin XL- fibrin degradation products (FDP) 39

Degradation of Fibrin/Fibrinogen or Fibrin Plasmin Fragment X Small Peptides Plasmin Fragment Y Fragment D Small Peptides Plasmin Fragment E Fragment D Small Peptides 40

Approach to evaluate Fibrinolysis D-Dimer, a measure of fibrin degradation products, is the final product formed during the fibrinolysis process by plasmin 41

Approach to evaluate Fibrinolysis cont, – Elevated levels of D-Dimer are indicative of on -going fibrinolysis • Found high in : • (DVT) • (PE. Pulmonary embolism) • (DIC) • D-Dimer levels also rise during the normal pregnancy and very high levels are associated with complications. 42

Bleeding Disorders 43

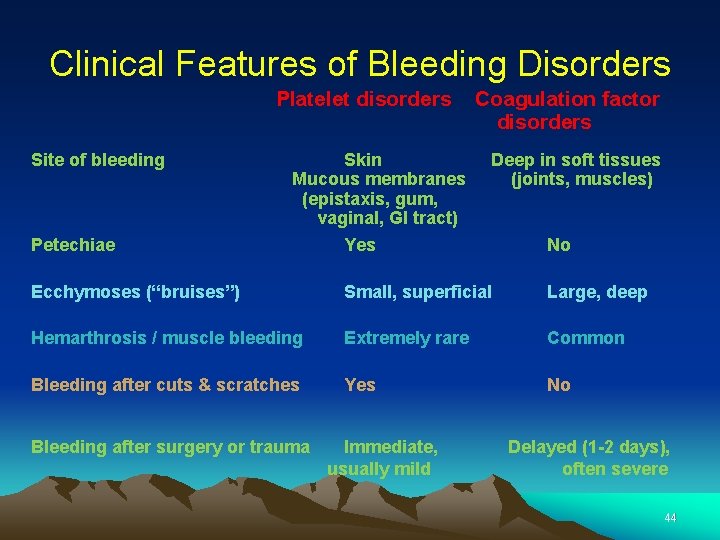
Clinical Features of Bleeding Disorders Platelet disorders Site of bleeding Petechiae Skin Mucous membranes (epistaxis, gum, vaginal, GI tract) Yes Coagulation factor disorders Deep in soft tissues (joints, muscles) No Ecchymoses (“bruises”) Small, superficial Large, deep Hemarthrosis / muscle bleeding Extremely rare Common Bleeding after cuts & scratches Yes No Bleeding after surgery or trauma Immediate, usually mild Delayed (1 -2 days), often severe 44

Hematologic disorders causing bleeding – Coagulation factor disorders – Platelet disorders 45

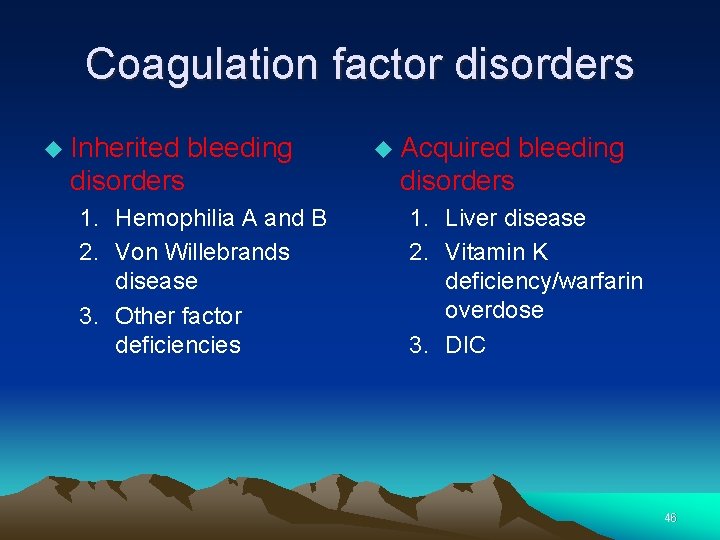
Coagulation factor disorders Inherited bleeding disorders 1. Hemophilia A and B 2. Von Willebrands disease 3. Other factor deficiencies Acquired bleeding disorders 1. Liver disease 2. Vitamin K deficiency/warfarin overdose 3. DIC 46

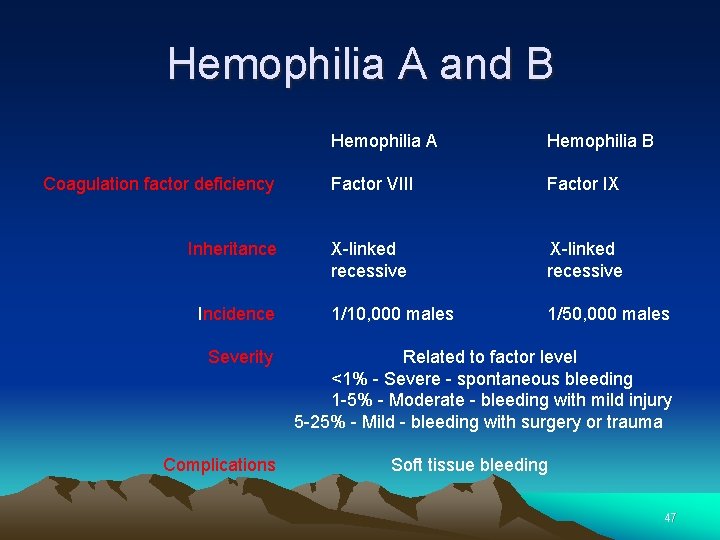
Hemophilia A and B Hemophilia A Hemophilia B Coagulation factor deficiency Factor VIII Factor IX Inheritance X-linked recessive Incidence 1/10, 000 males 1/50, 000 males Severity Related to factor level <1% - Severe - spontaneous bleeding 1 -5% - Moderate - bleeding with mild injury 5 -25% - Mild - bleeding with surgery or trauma Complications Soft tissue bleeding 47

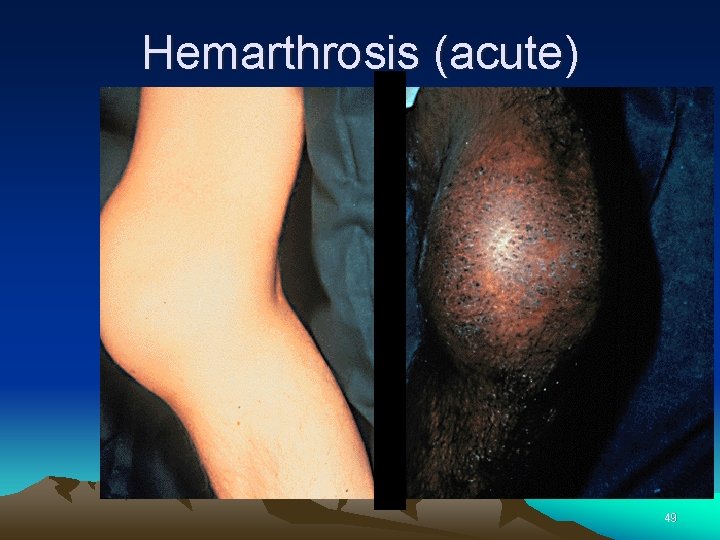
Hemophilia Clinical manifestations (hemophilia A & B are indistinguishable) Hemarthrosis (most common) Fixed joints Soft tissue hematomas (e. g. , muscle) Muscle atrophy Shortened tendons Other sites of bleeding Urinary tract CNS, neck (may be life-threatening) Prolonged bleeding after surgery or dental extractions 48

Hemarthrosis (acute) 49

von Willebrand Disease: Clinical Features von Willebrand factor – – – Synthesis in endothelium and megakaryocytes Forms large multimer Carrier of factor VIII Anchors platelets to subendothelium Bridge between platelets Inheritance - autosomal dominant Incidence - 1/10, 000 Clinical features - mucocutaneous bleeding 50

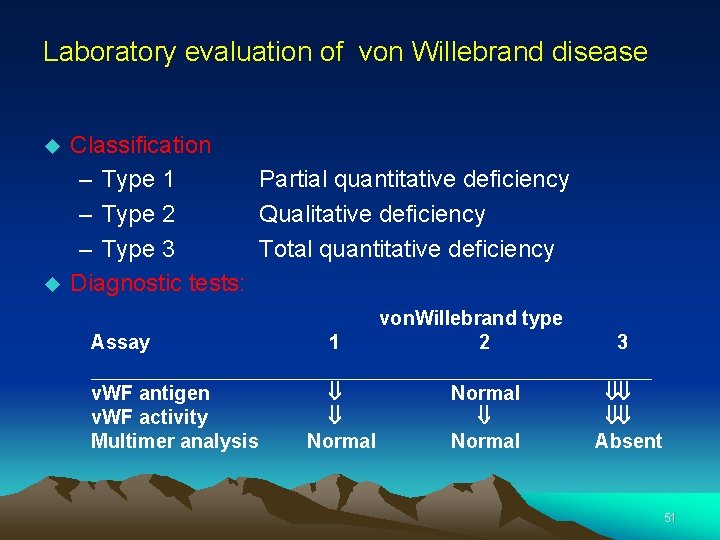
Laboratory evaluation of von Willebrand disease Classification – Type 1 Partial quantitative deficiency – Type 2 Qualitative deficiency – Type 3 Total quantitative deficiency Diagnostic tests: Assay 1 von. Willebrand type 2 3 __________________________ v. WF antigen v. WF activity Multimer analysis ß ß Normal ßß ßß Absent 51

Vitamin K deficiency Source of vitamin K Green vegetables Synthesized by intestinal flora Required for synthesis Factors II, VII, IX , X, Protein C and S Causes of deficiency Malnutrition Biliary obstruction Malabsorption Antibiotic therapy Treatment Vitamin K Fresh frozen plasma 52

Common clinical conditions associated with Disseminated Intravascular Coagulation • Activation of both coagulation and fibrinolysis • Sepsis • Trauma – Head injury – Fat embolism • Malignancy Triggered by • Obstetrical complications – Amniotic fluid embolism – Abruptio placentae • Vascular disorders • Reaction to toxin (e. g. snake venom, drugs) • Immunologic disorders – Severe allergic reaction – Transplant rejection 53

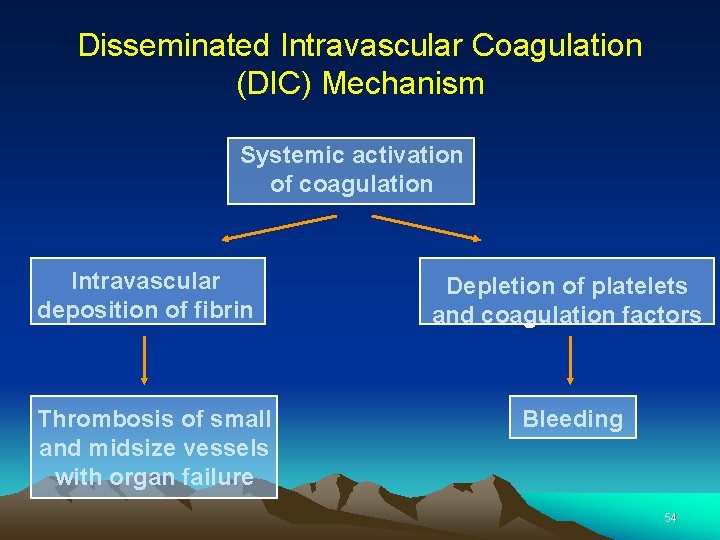
Disseminated Intravascular Coagulation (DIC) Mechanism Systemic activation of coagulation Intravascular deposition of fibrin Thrombosis of small and midsize vessels with organ failure Depletion of platelets and coagulation factors Bleeding 54

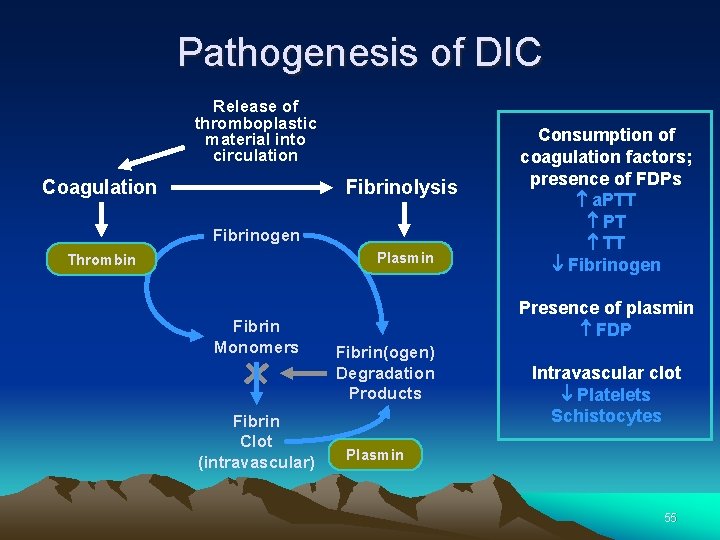
Pathogenesis of DIC Release of thromboplastic material into circulation Coagulation Fibrinolysis Fibrinogen Plasmin Thrombin Fibrin Monomers Fibrin Clot (intravascular) Consumption of coagulation factors; presence of FDPs a. PTT PT TT Fibrinogen Presence of plasmin FDP Fibrin(ogen) Degradation Products Intravascular clot Platelets Schistocytes Plasmin 55

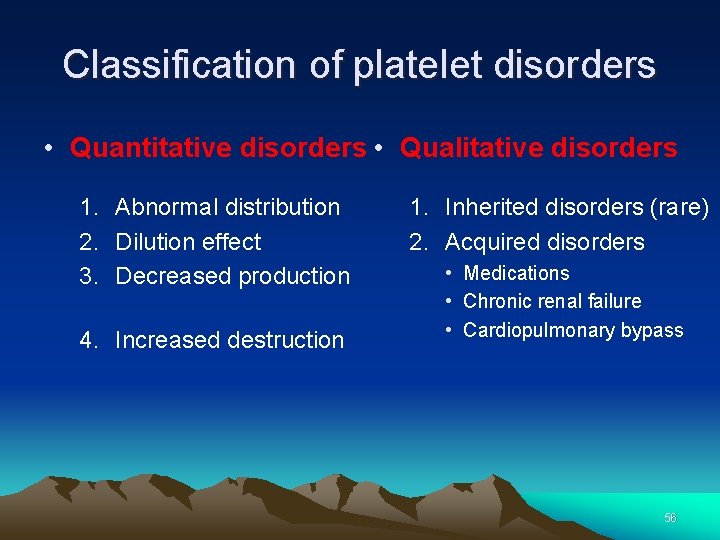
Classification of platelet disorders • Quantitative disorders • Qualitative disorders 1. Abnormal distribution 2. Dilution effect 3. Decreased production 4. Increased destruction 1. Inherited disorders (rare) 2. Acquired disorders • Medications • Chronic renal failure • Cardiopulmonary bypass 56

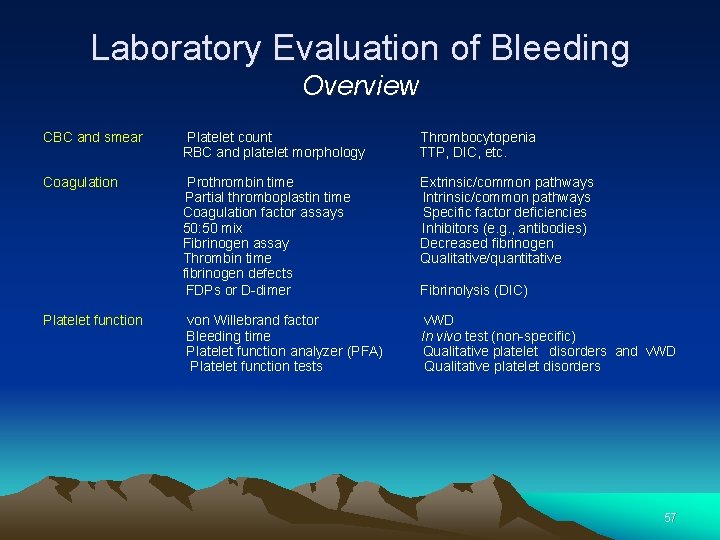
Laboratory Evaluation of Bleeding Overview CBC and smear Platelet count Thrombocytopenia RBC and platelet morphology TTP, DIC, etc. Coagulation Prothrombin time Extrinsic/common pathways Partial thromboplastin time Intrinsic/common pathways Coagulation factor assays Specific factor deficiencies 50: 50 mix Inhibitors (e. g. , antibodies) Fibrinogen assay Decreased fibrinogen Thrombin time Qualitative/quantitative fibrinogen defects FDPs or D-dimer Fibrinolysis (DIC) Platelet function von Willebrand factor v. WD Bleeding time In vivo test (non-specific) Platelet function analyzer (PFA) Qualitative platelet disorders and v. WD Platelet function tests Qualitative platelet disorders 57

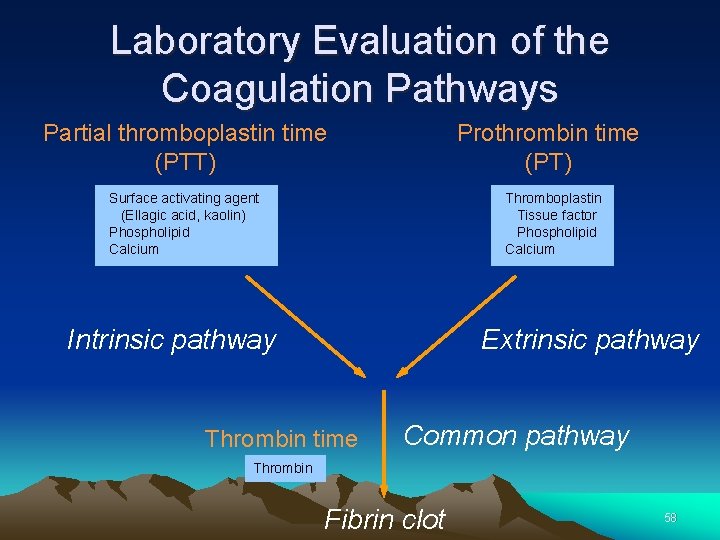
Laboratory Evaluation of the Coagulation Pathways Partial thromboplastin time (PTT) Prothrombin time (PT) Surface activating agent (Ellagic acid, kaolin) Phospholipid Calcium Thromboplastin Tissue factor Phospholipid Calcium Intrinsic pathway Extrinsic pathway Thrombin time Common pathway Thrombin Fibrin clot 58

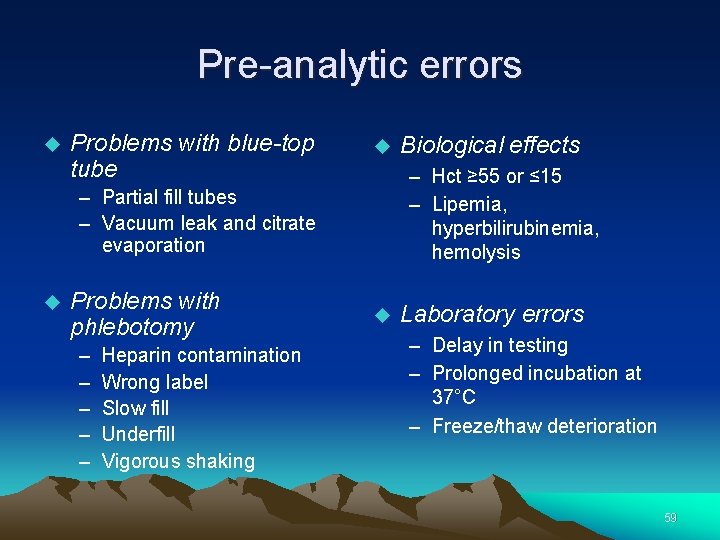
Pre-analytic errors Problems with blue-top tube – Hct ≥ 55 or ≤ 15 – Lipemia, hyperbilirubinemia, hemolysis – Partial fill tubes – Vacuum leak and citrate evaporation Problems with phlebotomy – – – Heparin contamination Wrong label Slow fill Underfill Vigorous shaking Biological effects Laboratory errors – Delay in testing – Prolonged incubation at 37°C – Freeze/thaw deterioration 59

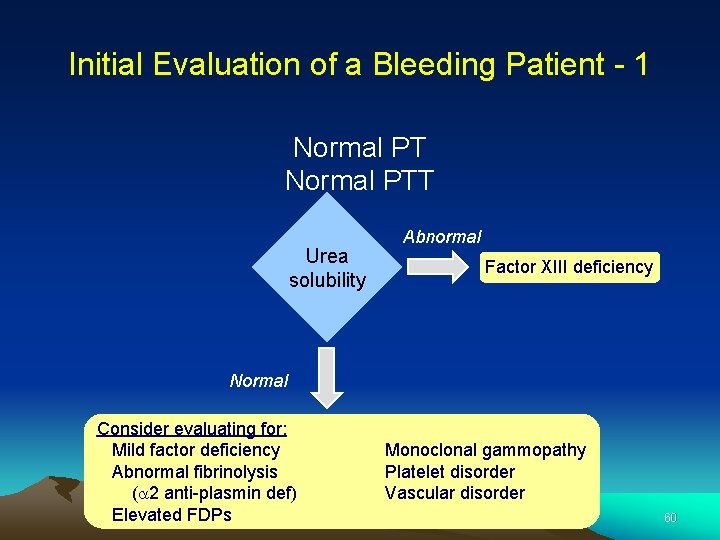
Initial Evaluation of a Bleeding Patient - 1 Normal PTT Urea solubility Abnormal Factor XIII deficiency Normal Consider evaluating for: Mild factor deficiency Abnormal fibrinolysis (a 2 anti-plasmin def) Elevated FDPs Monoclonal gammopathy Platelet disorder Vascular disorder 60

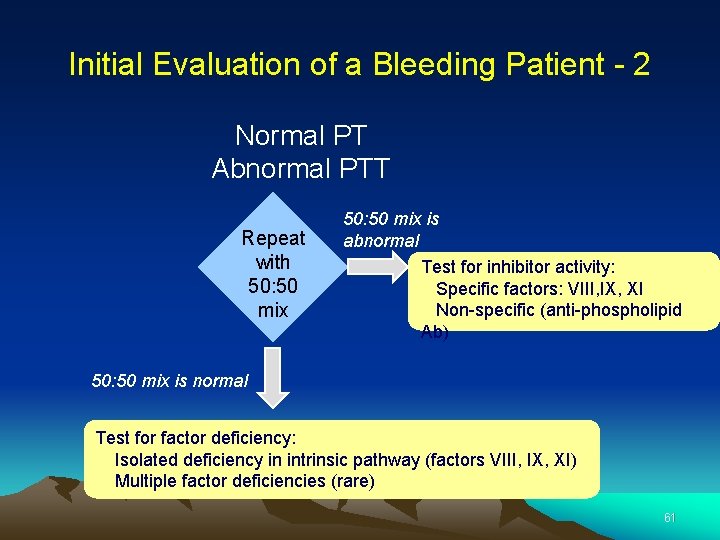
Initial Evaluation of a Bleeding Patient - 2 Normal PT Abnormal PTT Repeat with 50: 50 mix is abnormal Test for inhibitor activity: Specific factors: VIII, IX, XI Non-specific (anti-phospholipid Ab) 50: 50 mix is normal Test for factor deficiency: Isolated deficiency in intrinsic pathway (factors VIII, IX, XI) Multiple factor deficiencies (rare) 61

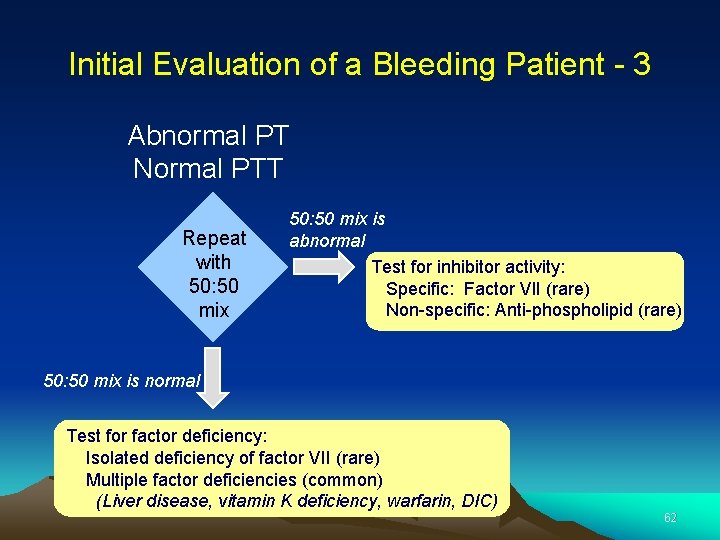
Initial Evaluation of a Bleeding Patient - 3 Abnormal PT Normal PTT Repeat with 50: 50 mix is abnormal Test for inhibitor activity: Specific: Factor VII (rare) Non-specific: Anti-phospholipid (rare) 50: 50 mix is normal Test for factor deficiency: Isolated deficiency of factor VII (rare) Multiple factor deficiencies (common) (Liver disease, vitamin K deficiency, warfarin, DIC) 62

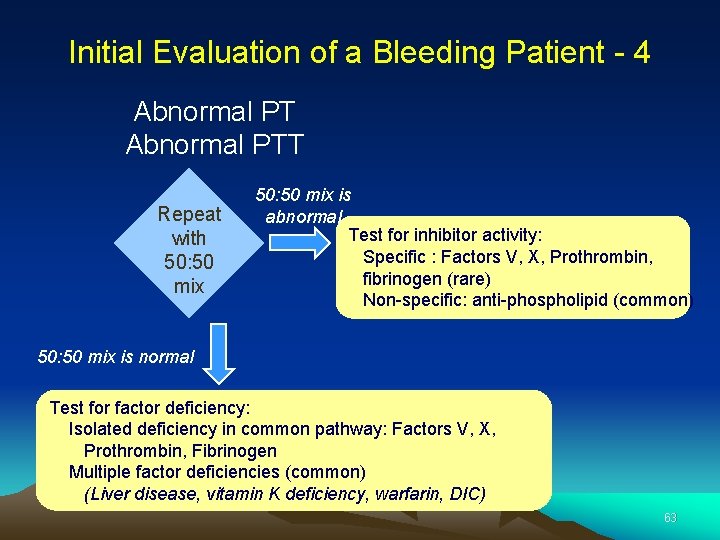
Initial Evaluation of a Bleeding Patient - 4 Abnormal PTT Repeat with 50: 50 mix is abnormal Test for inhibitor activity: Specific : Factors V, X, Prothrombin, fibrinogen (rare) Non-specific: anti-phospholipid (common) 50: 50 mix is normal Test for factor deficiency: Isolated deficiency in common pathway: Factors V, X, Prothrombin, Fibrinogen Multiple factor deficiencies (common) (Liver disease, vitamin K deficiency, warfarin, DIC) 63

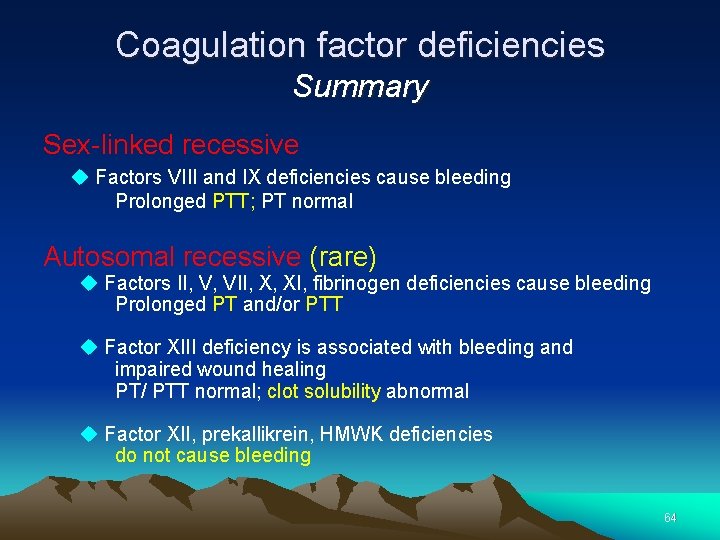
Coagulation factor deficiencies Summary Sex-linked recessive Factors VIII and IX deficiencies cause bleeding Prolonged PTT; PT normal Autosomal recessive (rare) Factors II, V, VII, X, XI, fibrinogen deficiencies cause bleeding Prolonged PT and/or PTT Factor XIII deficiency is associated with bleeding and impaired wound healing PT/ PTT normal; clot solubility abnormal Factor XII, prekallikrein, HMWK deficiencies do not cause bleeding 64

Thrombin Time Bypasses factors II-XII Measures rate of fibrinogen conversion to fibrin Procedure: – Add thrombin with patient plasma – Measure time to clot Variables: – Source and quantity of thrombin 65

Causes of prolonged Thrombin Time Heparin Hypofibrinogenemia Dysfibrinogenemia Elevated FDPs or paraprotein Thrombin inhibitors (Hirudin) Thrombin antibodies 66

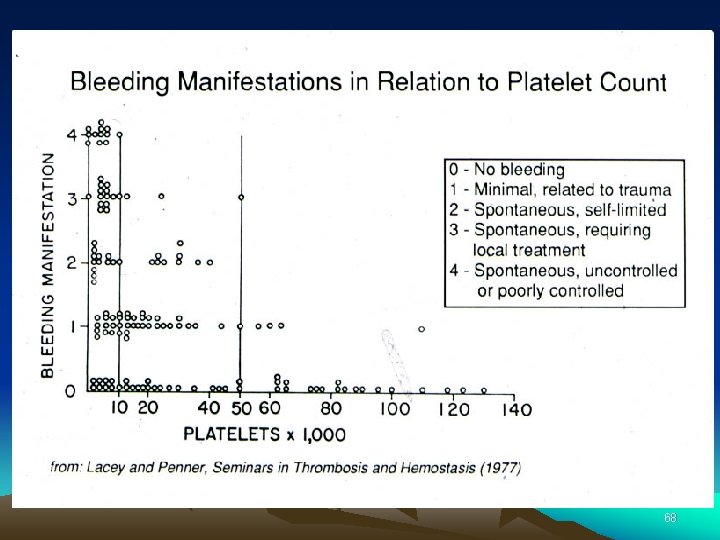
Approach to the thrombocytopenic patient History – Is the patient bleeding? – Are there symptoms of a secondary illness? (neoplasm, infection, autoimmune disease) – Is there a history of medications, alcohol use, or recent transfusion? – Are there risk factors for HIV infection? – Is there a family history of thrombocytopenia? – Do the sites of bleeding suggest a platelet defect? Assess the number and function of platelets – CBC with peripheral smear – Bleeding time or platelet aggregation study 67

68

Bleeding time and bleeding 5 -10% of patients have a prolonged bleeding time Most of the prolonged bleeding times are due to aspirin or drug ingestion Prolonged bleeding time does not predict excess surgical blood loss Not recommended for routine testing in preoperative patients 69

Conclusion Special Coagulation is a specialized, complex and dynamic field! 70
 Fc silmi
Fc silmi Introduction of hematology
Introduction of hematology Electrodes used in surgical diathermy
Electrodes used in surgical diathermy Disseminated intravascular coagulation pathophysiology
Disseminated intravascular coagulation pathophysiology Clotting time capillary method
Clotting time capillary method Coagulation made easy
Coagulation made easy Coagulation profile test
Coagulation profile test Pycnose reversible
Pycnose reversible Vasoconstriction
Vasoconstriction Coagulation factors
Coagulation factors Gelatinisation
Gelatinisation Egg coagulate
Egg coagulate Coagulation profile test
Coagulation profile test Plasma and serum
Plasma and serum Coagulation casecade
Coagulation casecade D'alessio
D'alessio Dvt workbook
Dvt workbook Bernard soulier syndrome
Bernard soulier syndrome Coagulation eggs definition
Coagulation eggs definition Coagulation cascade
Coagulation cascade Dicoumoral
Dicoumoral Blood clotting mechanism
Blood clotting mechanism Disseminated intravascular coagulation
Disseminated intravascular coagulation Unc benign hematology
Unc benign hematology Hematology laboratory procedures
Hematology laboratory procedures Reticulocyte count stain
Reticulocyte count stain Reticulocyte index formula
Reticulocyte index formula Kusum viswanathan
Kusum viswanathan Blok diagram hematology analyzer
Blok diagram hematology analyzer Premier oncology hematology management society
Premier oncology hematology management society Hretic
Hretic Sucking tube in hematology
Sucking tube in hematology Hematology medical terminology
Hematology medical terminology Lsu hematology oncology fellowship
Lsu hematology oncology fellowship American society of hematology
American society of hematology American society of hematology
American society of hematology Diagnostic suffixes
Diagnostic suffixes Retic count
Retic count Hematology
Hematology Glicemide
Glicemide Hemolyser
Hemolyser Medical student hematology lectures
Medical student hematology lectures Nfs reticulocytes
Nfs reticulocytes Practical hematology
Practical hematology Hypochromic red cells
Hypochromic red cells Hematology
Hematology Branches of hematology
Branches of hematology Wbc differential
Wbc differential Dr. biren saraiya hematology
Dr. biren saraiya hematology Unit 3 abbreviations medical terminology
Unit 3 abbreviations medical terminology Haemato oncology wiki
Haemato oncology wiki Hematology day
Hematology day Uhs hematology
Uhs hematology Umkc hematology oncology fellowship
Umkc hematology oncology fellowship Antitussives
Antitussives Aligarh movement
Aligarh movement Zak ahmad
Zak ahmad Dr fawad randhawa
Dr fawad randhawa Jawad ahmad md
Jawad ahmad md Nur ahmad husin
Nur ahmad husin Gayatiri
Gayatiri Dr angel rodriguez-chevres
Dr angel rodriguez-chevres Dr usama ahmad
Dr usama ahmad Dr nur ahmad tabri
Dr nur ahmad tabri Nur ahmad husin
Nur ahmad husin Nur ahmad husin
Nur ahmad husin Ahmad ibrahim primary school review
Ahmad ibrahim primary school review Ahmad boestamam sejarah tingkatan 4
Ahmad boestamam sejarah tingkatan 4 Siapakah pengasas pergerakan pengakap di malaysia
Siapakah pengasas pergerakan pengakap di malaysia Econ 25
Econ 25 Hadi ahmad md
Hadi ahmad md