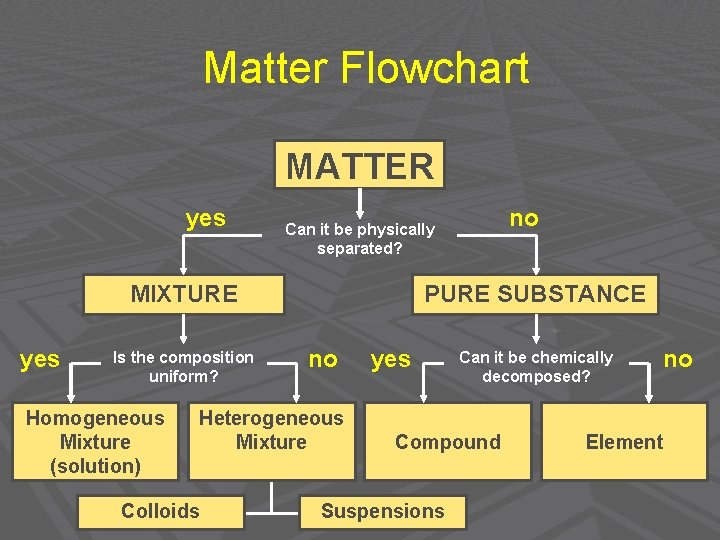
Introduction to Solutions Matter Flowchart MATTER yes MIXTURE

















- Slides: 37

Introduction to Solutions

Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element

Pure Substances • A pure substance has a definite composition. • Pure substances can be elements or compounds

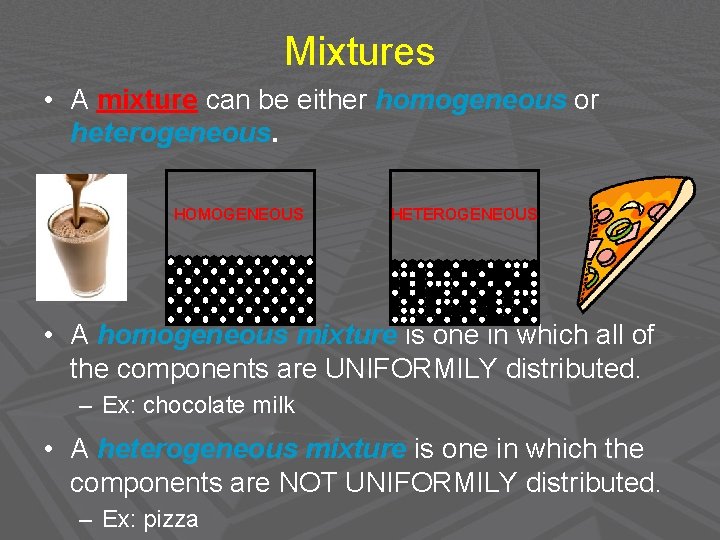
Mixtures • A mixture can be either homogeneous or heterogeneous. HOMOGENEOUS HETEROGENEOUS • A homogeneous mixture is one in which all of the components are UNIFORMILY distributed. – Ex: chocolate milk • A heterogeneous mixture is one in which the components are NOT UNIFORMILY distributed. – Ex: pizza

Solutions, in chemistry, are homogeneous mixtures of two or more substances. The substance present in largest quantity usually is called the solvent. The solvent can be either a liquid or a solid. The substance that is present in smallest quantity is said to be dissolved and is called the solute. The solute can be either a gas, a liquid, or a solid.

Concept Check Coke lists as its ingredients as: “carbonated water, high fructose corn syrup and/or sucrose, caramel color, phosphoric acid, natural flavors, caffeine”. What is the solvent? What are the solutes? What can we classify CO 2 as in carbonated beverages?

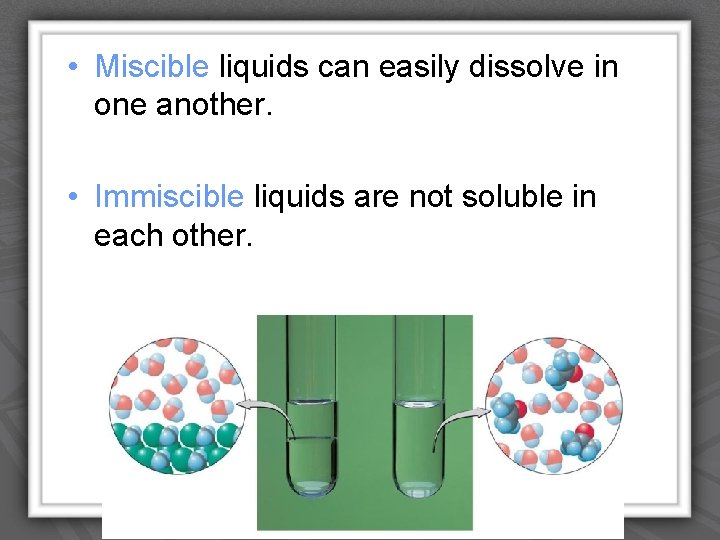
• Miscible liquids can easily dissolve in one another. • Immiscible liquids are not soluble in each other. IPC-Solutions-Borders

Heterogeneous Mixtures v. Suspensions-a heterogeneous mixture that contains large particles that “settle out” unless constantly stirred or agitated v. Ex: freshly squeezed OJ, salad dressing v. Colloids-a heterogeneous mixture in which the components are microscopic and will not separate when left standing. v. Ex: mayonnaise, milk, stick deodorant

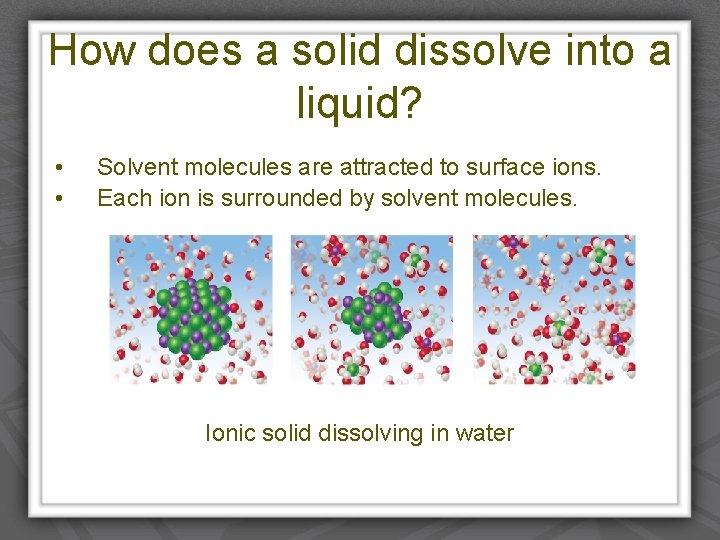
How does a solid dissolve into a liquid? • • Solvent molecules are attracted to surface ions. Each ion is surrounded by solvent molecules. Ionic solid dissolving in water

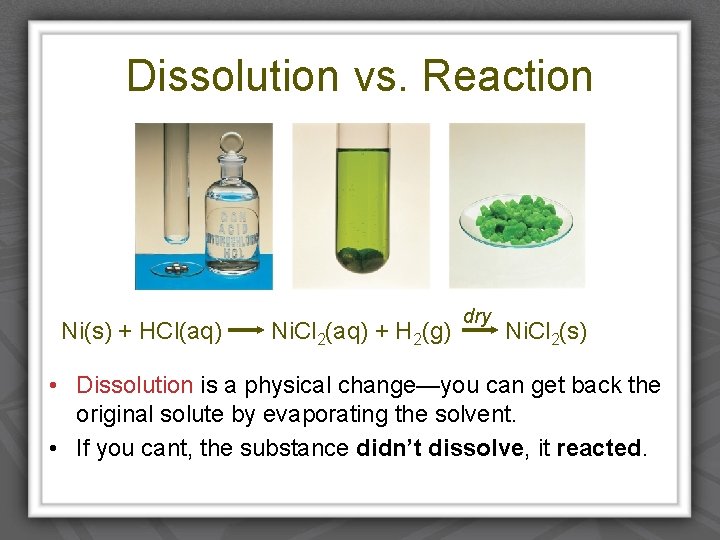
Dissolution vs. Reaction Ni(s) + HCl(aq) Ni. Cl 2(aq) + H 2(g) dry Ni. Cl 2(s) • Dissolution is a physical change—you can get back the original solute by evaporating the solvent. • If you cant, the substance didn’t dissolve, it reacted.

Factors Affecting Solubility • Chemists use the saying “like dissolves like: ” Ø Polar substances tend to dissolve in polar solvents. Ø Nonpolar substances tend to dissolve in nonpolar solvents. Oil is nonpolar while water is polar. They are immiscible.

Saturation Types • Saturated • Unsaturated

Degree of saturation • Unsaturated Solution Ø Less than the maximum amount of solute for that temperature is dissolved in the solvent. Ø No solid remains in flask.

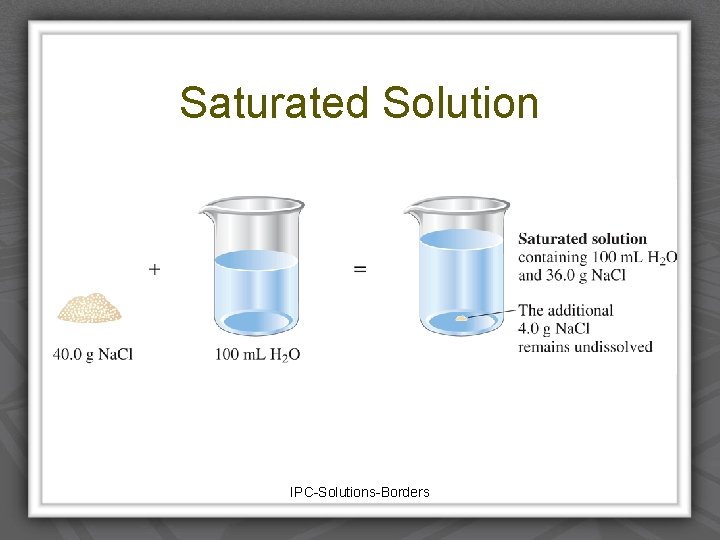
Degree of saturation • Saturated solution Ø Solvent holds as much solute as is possible at that temperature. Ø Undissolved solid remains in flask. Ø Dissolved solute is in dynamic equilibrium with solid solute particles.

Saturated Solution IPC-Solutions-Borders

Degree of saturation • Supersaturated Solution Ø Solvent holds more solute than is normally possible at that temperature. Ø These solutions are unstable

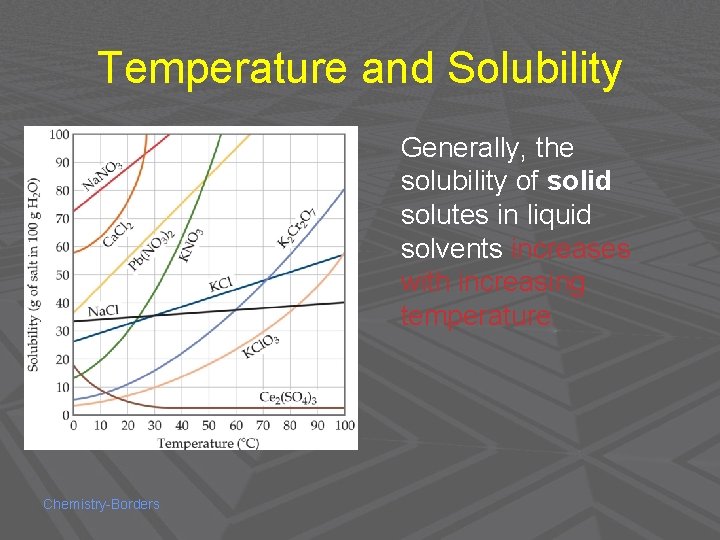
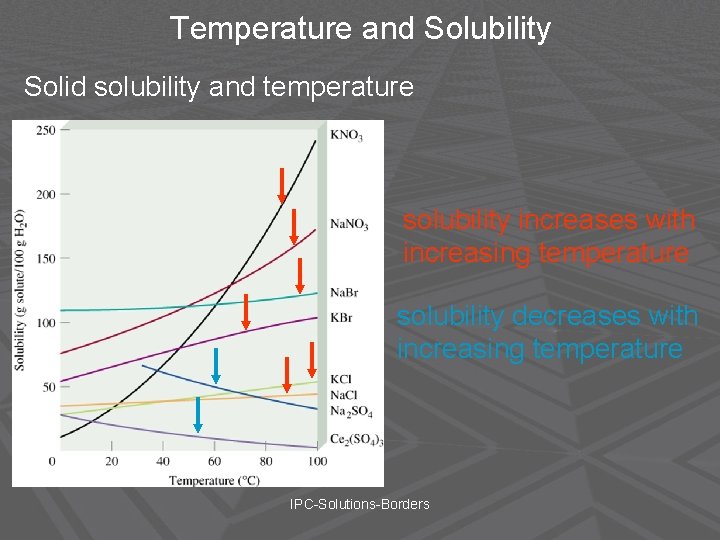
Temperature and Solubility Generally, the solubility of solid solutes in liquid solvents increases with increasing temperature. Chemistry-Borders

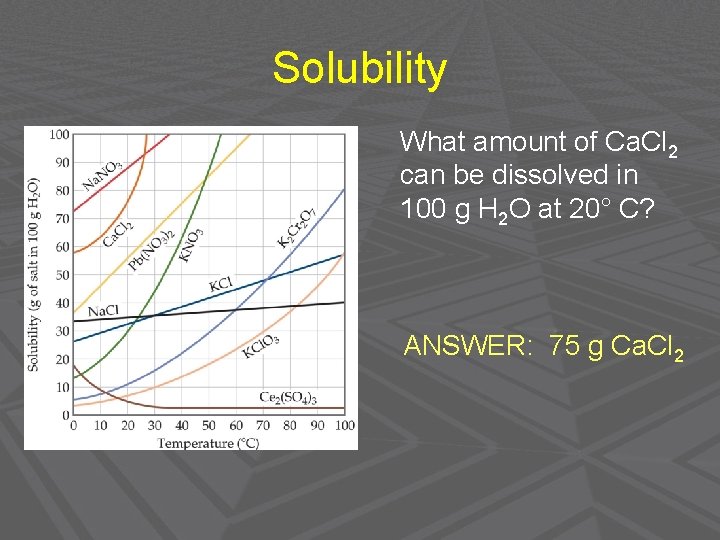
Solubility What amount of Ca. Cl 2 can be dissolved in 100 g H 2 O at 20° C? ANSWER: 75 g Ca. Cl 2

Let’s play a quick game of…. Saturated or Unsaturated? Chemistry-Borders IPC-Solutions-Borders

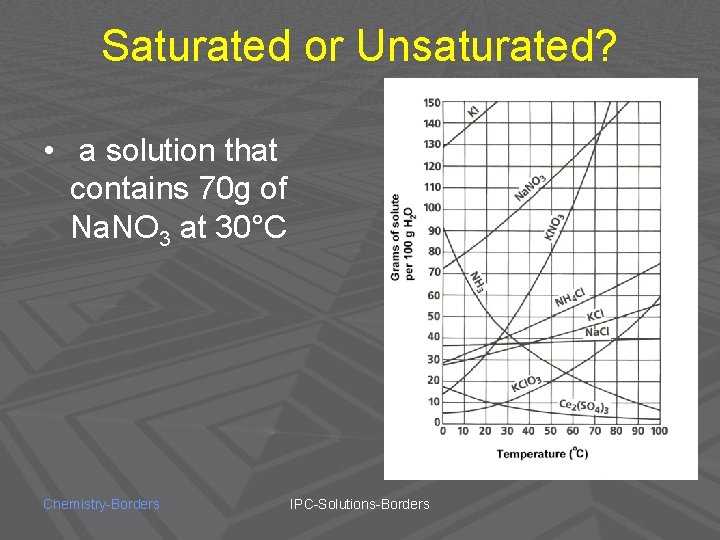
Saturated or Unsaturated? • a solution that contains 70 g of Na. NO 3 at 30°C Chemistry-Borders IPC-Solutions-Borders

UNSATURATED! Chemistry-Borders IPC-Solutions-Borders

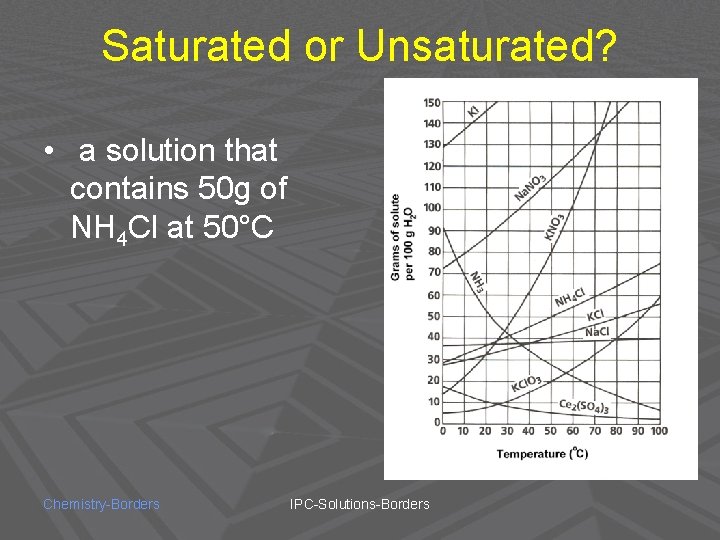
Saturated or Unsaturated? • a solution that contains 50 g of NH 4 Cl at 50°C Chemistry-Borders IPC-Solutions-Borders

SATURATED! Chemistry-Borders IPC-Solutions-Borders

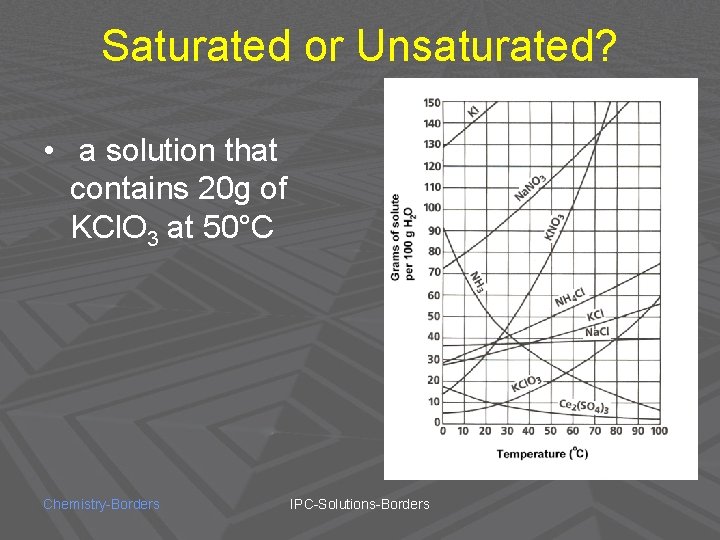
Saturated or Unsaturated? • a solution that contains 20 g of KCl. O 3 at 50°C Chemistry-Borders IPC-Solutions-Borders

SATURATED! Chemistry-Borders IPC-Solutions-Borders

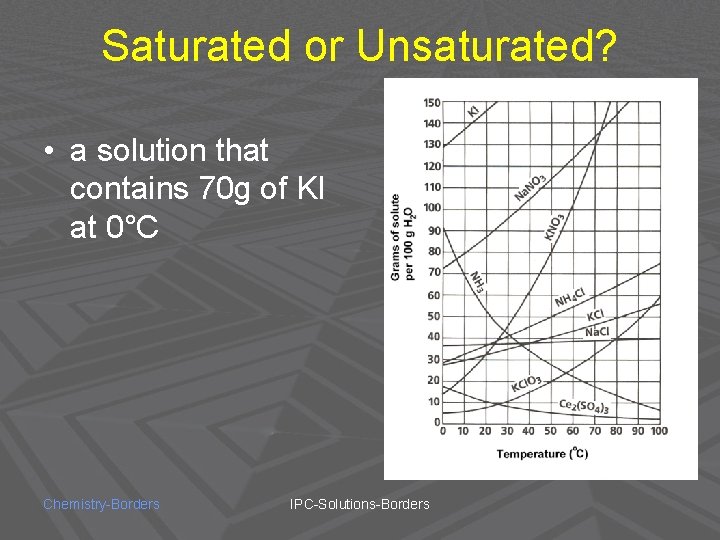
Saturated or Unsaturated? • a solution that contains 70 g of KI at 0°C Chemistry-Borders IPC-Solutions-Borders

UNSATURATED! Chemistry-Borders IPC-Solutions-Borders

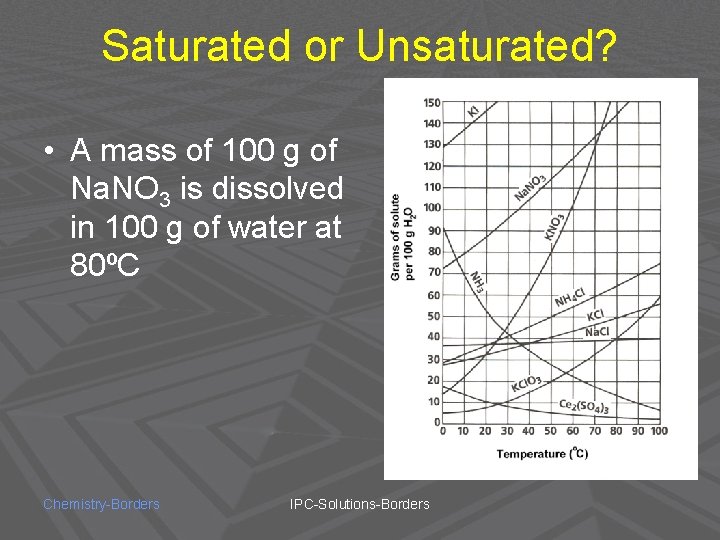
Saturated or Unsaturated? • A mass of 100 g of Na. NO 3 is dissolved in 100 g of water at 80ºC Chemistry-Borders IPC-Solutions-Borders

UNSATURATED! Chemistry-Borders IPC-Solutions-Borders

THE END Chemistry-Borders IPC-Solutions-Borders

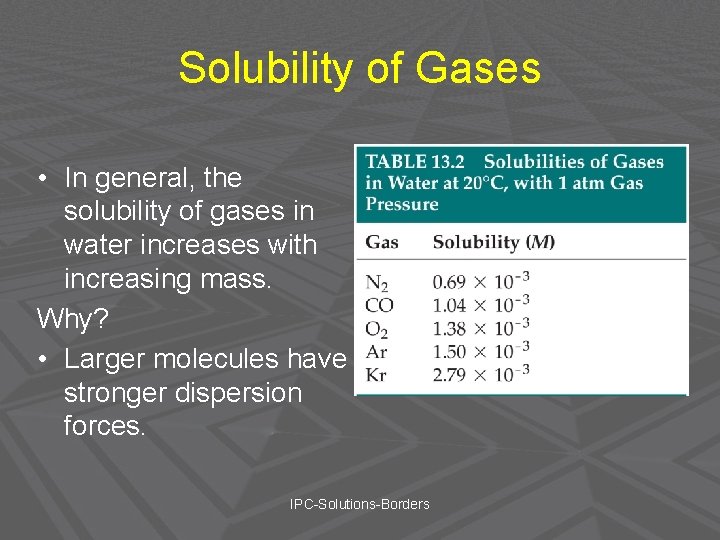
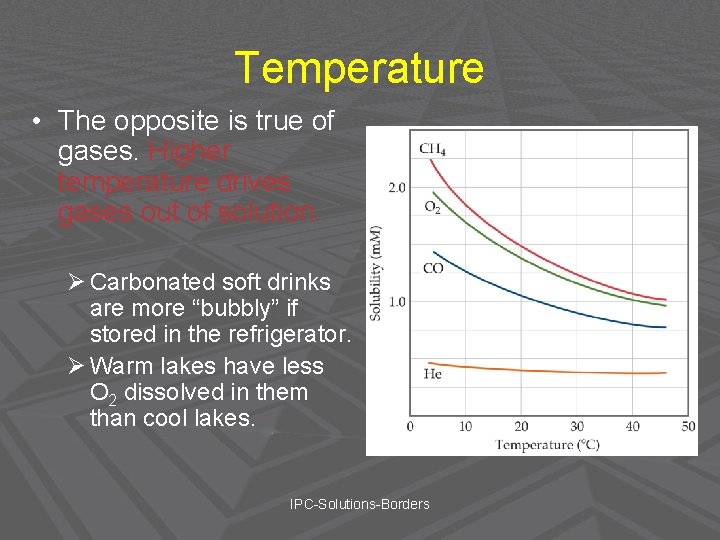
Solubility of Gases • In general, the solubility of gases in water increases with increasing mass. Why? • Larger molecules have stronger dispersion forces. IPC-Solutions-Borders

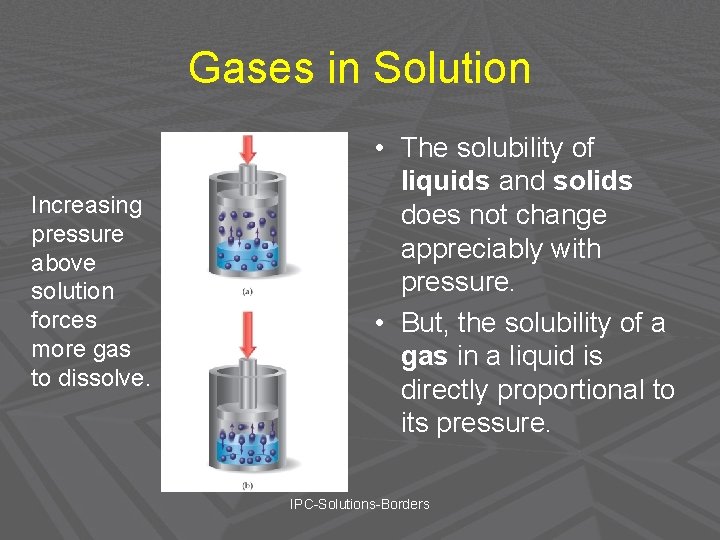
Gases in Solution Increasing pressure above solution forces more gas to dissolve. • The solubility of liquids and solids does not change appreciably with pressure. • But, the solubility of a gas in a liquid is directly proportional to its pressure. IPC-Solutions-Borders

Temperature • The opposite is true of gases. Higher temperature drives gases out of solution. Ø Carbonated soft drinks are more “bubbly” if stored in the refrigerator. Ø Warm lakes have less O 2 dissolved in them than cool lakes. IPC-Solutions-Borders

Temperature and Solubility Solid solubility and temperature solubility increases with increasing temperature solubility decreases with increasing temperature IPC-Solutions-Borders

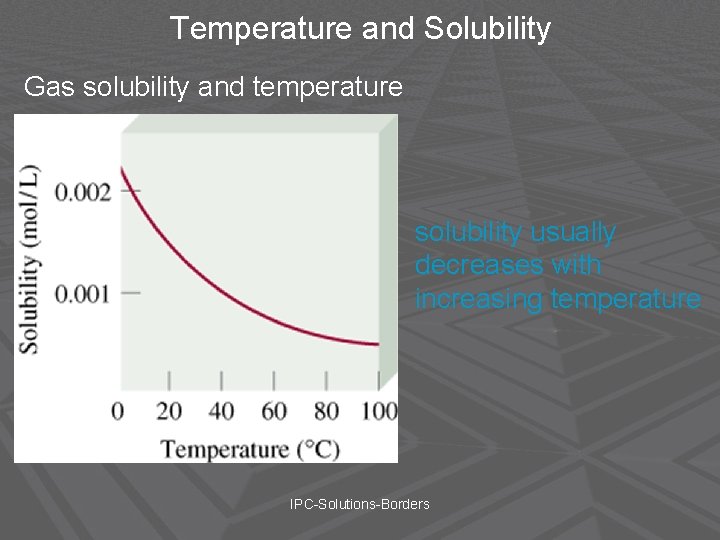
Temperature and Solubility Gas solubility and temperature solubility usually decreases with increasing temperature IPC-Solutions-Borders

Electrolytes IPC-Solutions-Borders

Electrolyte: a substance that dissolves in water to give a solution that conducts electric current • Any soluble ionic compound is an electrolyte • Strong acids are electrolytes IPC-Solutions-Borders