Introduction to Solutions LACC Chem 101 1 Properties



![p. H of Solutions p. H = - log [H 3 O+] p. H p. H of Solutions p. H = - log [H 3 O+] p. H](https://slidetodoc.com/presentation_image_h2/48cc0d6789519ed8c6cbeb6d411fbe36/image-20.jpg)
- Slides: 21

Introduction to Solutions LACC Chem 101 1

Properties of Solutions 1. A solution is composed of: ¡ solute: the minor component (least number of moles) ¡ solvent: the major component (largest number of moles) 2. Solubility: ¡ ¡ A soluble substance readily dissolves in the solvent. An insoluble substance will NOT dissolve readily in a solvent. 3. Miscibility: ¡ Two liquids are miscible in each other if they readily mix to form a uniform solution. Two immiscible liquids will always separate out into two distinct layers. 4. Solubility ¡ describes the amount of solute that will dissolve in a solvent. For example, 35. 7 g of Na. Cl will dissolve in 100 m. L of water at 0 o. C , no more. LACC Chem 101 2

General Properties of Solutions 3 1. Homogeneous mixture of two or more components. 2. Variable composition. 3. Solute is molecular or ionic in size. 4. May be either colored or colorless ¡ generally transparent 5. Solute remains uniformly distributed throughout the solution ¡will not settle out through time 6. The solute can be separated from the solvent by physical methods. LACC Chem 101

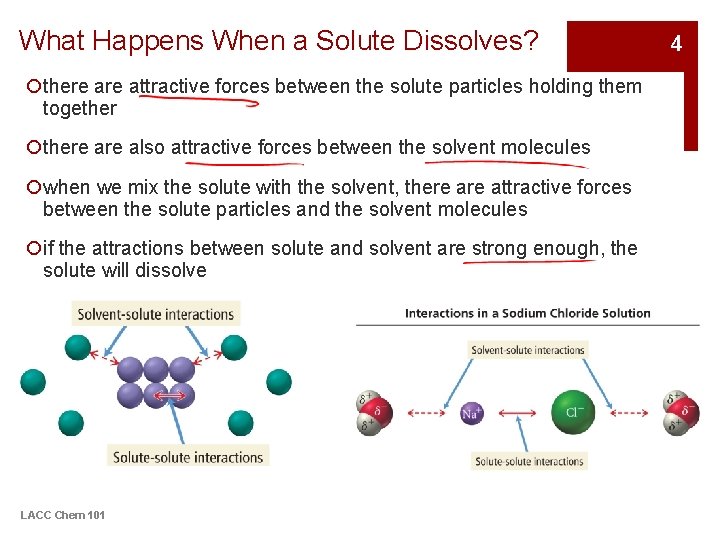
What Happens When a Solute Dissolves? ¡there attractive forces between the solute particles holding them together ¡there also attractive forces between the solvent molecules ¡when we mix the solute with the solvent, there attractive forces between the solute particles and the solvent molecules ¡if the attractions between solute and solvent are strong enough, the solute will dissolve LACC Chem 101 4

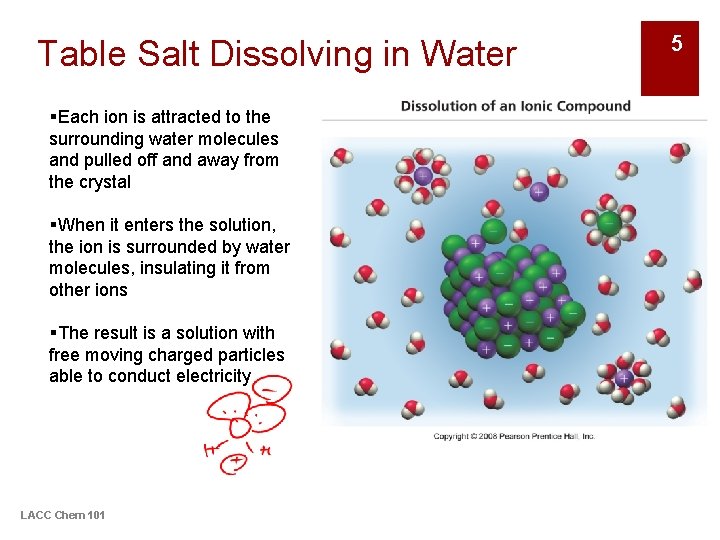
Table Salt Dissolving in Water §Each ion is attracted to the surrounding water molecules and pulled off and away from the crystal §When it enters the solution, the ion is surrounded by water molecules, insulating it from other ions §The result is a solution with free moving charged particles able to conduct electricity LACC Chem 101 5

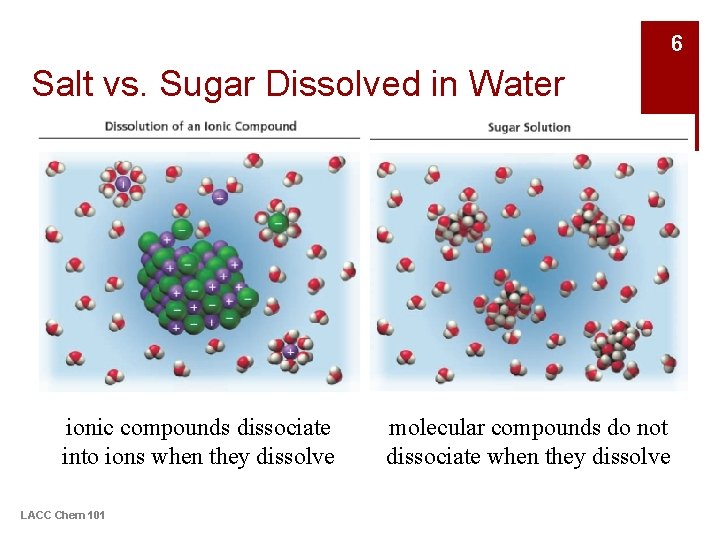
6 Salt vs. Sugar Dissolved in Water ionic compounds dissociate into ions when they dissolve LACC Chem 101 molecular compounds do not dissociate when they dissolve

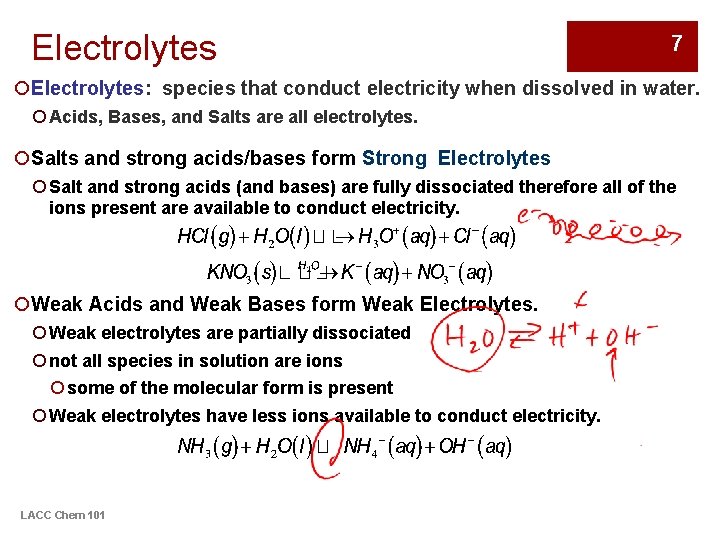
Electrolytes 7 ¡Electrolytes: species that conduct electricity when dissolved in water. ¡ Acids, Bases, and Salts are all electrolytes. ¡Salts and strong acids/bases form Strong Electrolytes ¡ Salt and strong acids (and bases) are fully dissociated therefore all of the ions present are available to conduct electricity. ¡Weak Acids and Weak Bases form Weak Electrolytes. ¡ Weak electrolytes are partially dissociated ¡ not all species in solution are ions ¡ some of the molecular form is present ¡ Weak electrolytes have less ions available to conduct electricity. LACC Chem 101

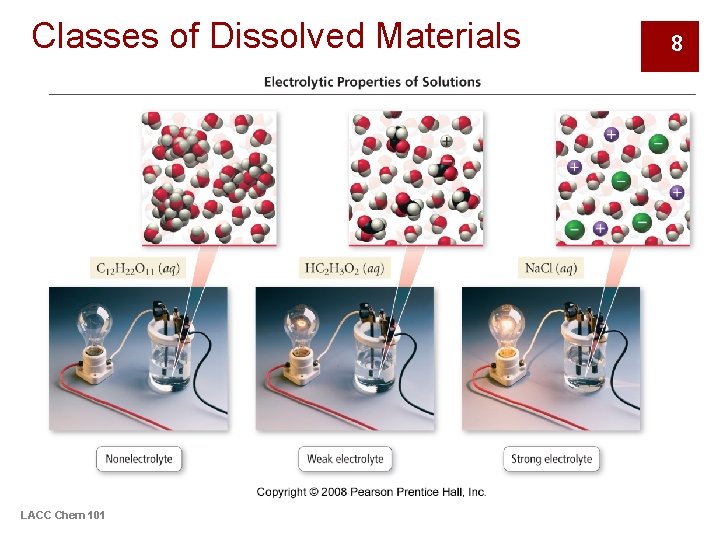
Classes of Dissolved Materials LACC Chem 101 8

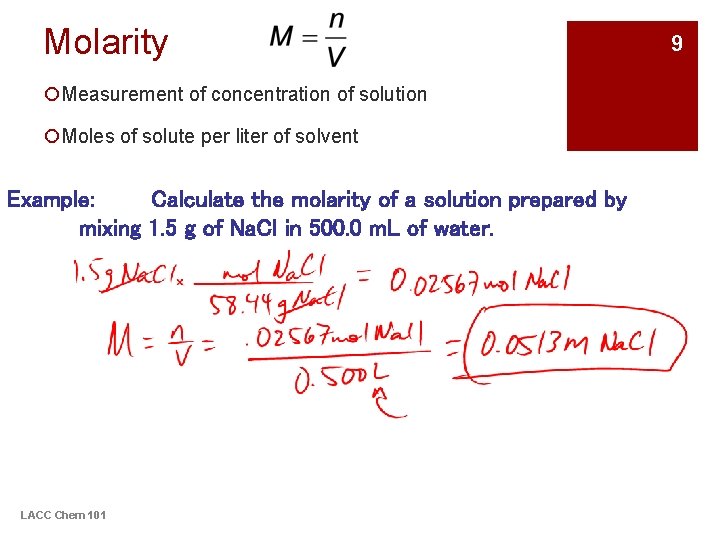
Molarity ¡Measurement of concentration of solution ¡Moles of solute per liter of solvent Example: Calculate the molarity of a solution prepared by mixing 1. 5 g of Na. Cl in 500. 0 m. L of water. LACC Chem 101 9

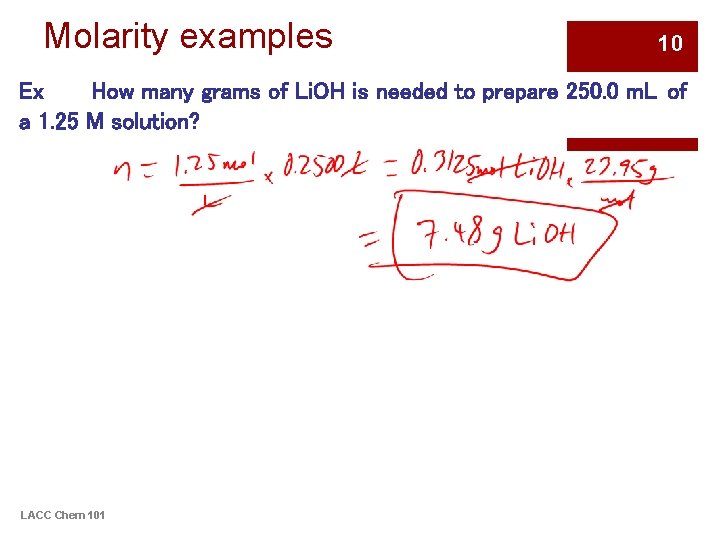
Molarity examples 10 Ex How many grams of Li. OH is needed to prepare 250. 0 m. L of a 1. 25 M solution? LACC Chem 101

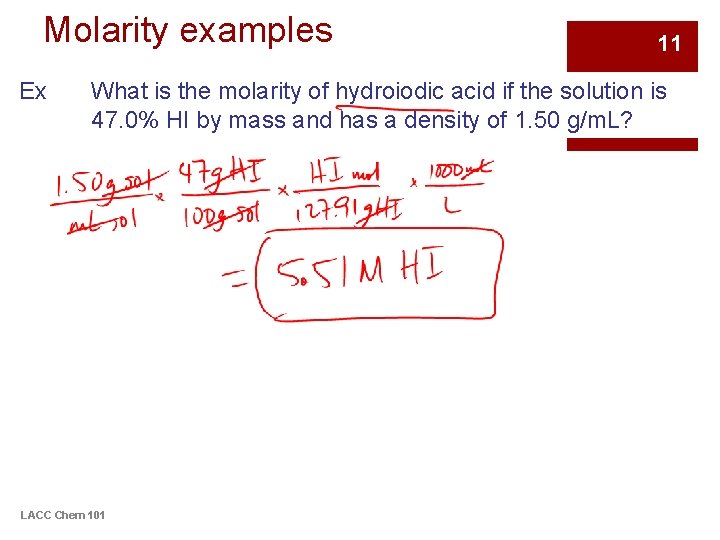
Molarity examples Ex 11 What is the molarity of hydroiodic acid if the solution is 47. 0% HI by mass and has a density of 1. 50 g/m. L? LACC Chem 101

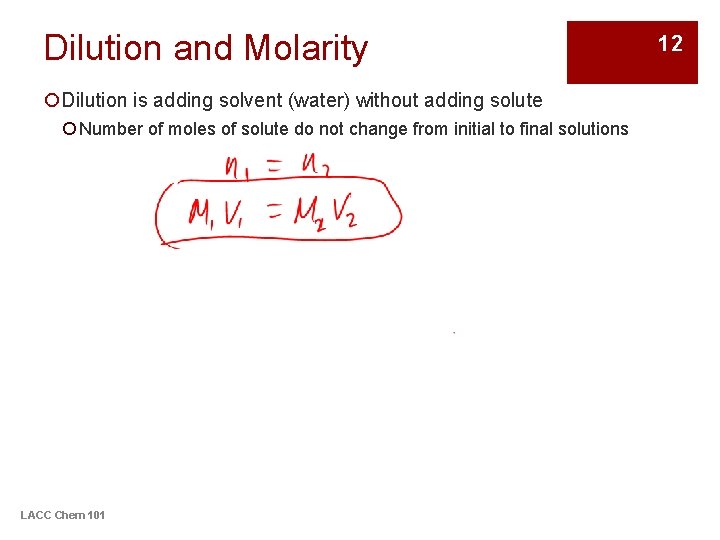
Dilution and Molarity ¡Dilution is adding solvent (water) without adding solute ¡ Number of moles of solute do not change from initial to final solutions LACC Chem 101 12

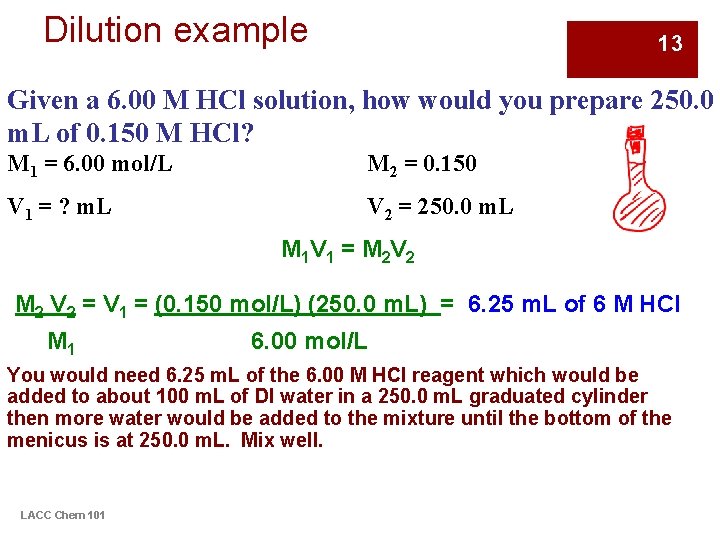
Dilution example 13 Given a 6. 00 M HCl solution, how would you prepare 250. 0 m. L of 0. 150 M HCl? M 1 = 6. 00 mol/L M 2 = 0. 150 V 1 = ? m. L V 2 = 250. 0 m. L M 1 V 1 = M 2 V 2 = V 1 = (0. 150 mol/L) (250. 0 m. L) = 6. 25 m. L of 6 M HCl M 1 6. 00 mol/L You would need 6. 25 m. L of the 6. 00 M HCl reagent which would be added to about 100 m. L of DI water in a 250. 0 m. L graduated cylinder then more water would be added to the mixture until the bottom of the menicus is at 250. 0 m. L. Mix well. LACC Chem 101

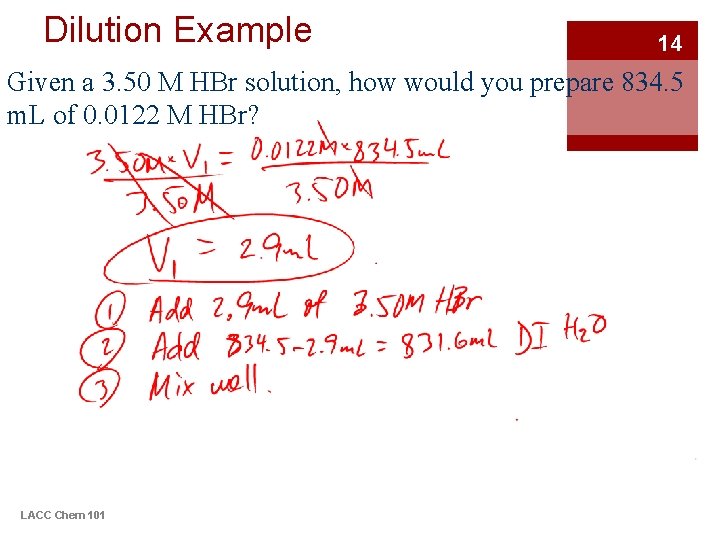
Dilution Example 14 Given a 3. 50 M HBr solution, how would you prepare 834. 5 m. L of 0. 0122 M HBr? LACC Chem 101

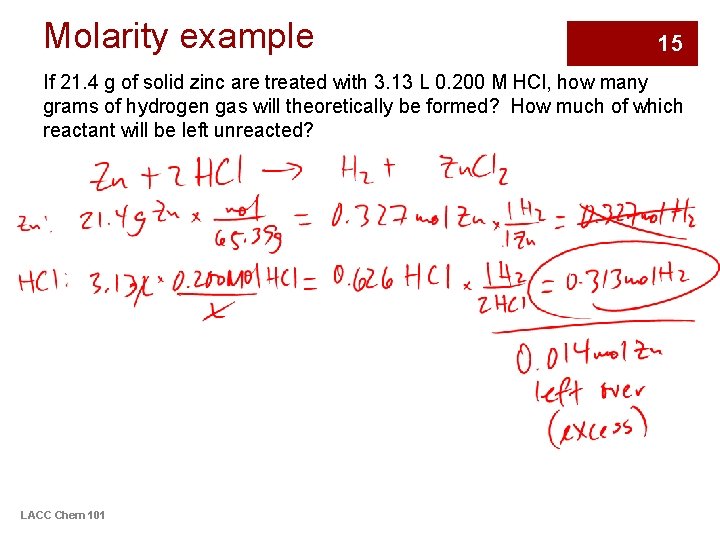
Molarity example 15 If 21. 4 g of solid zinc are treated with 3. 13 L 0. 200 M HCl, how many grams of hydrogen gas will theoretically be formed? How much of which reactant will be left unreacted? LACC Chem 101

Workshop on Molarity 16 1. Calculate the molarity of a solution made by dissolving 23. 4 g of sodium sulfate in enough water to form 125 m. L of solution. 2. Calculate the molarity of a solution made by dissolving 5. 00 g of glucose in sufficient water to form 100 m. L of solution. 3. A stock solution of 3. 0 M H 2 SO 4 is being used to make 500 m. L of 0. 10 M H 2 SO 4. How much water must be added to the more concentrated solution to make the less concentrated solution? 4. Calcium sulfide can be made by heating calcium sulfate with charcoal at high temperature according to the following unbalanced chemical equation: Ca. SO 4(s) + C(s) --> Ca. S(s) + CO(g) How many grams of Ca. S(s) can be prepared from 100. 0 g each of Ca. SO 4(s) and C(s)? How many grams of unreacted reactant remain at the end of this reaction? LACC Chem 101

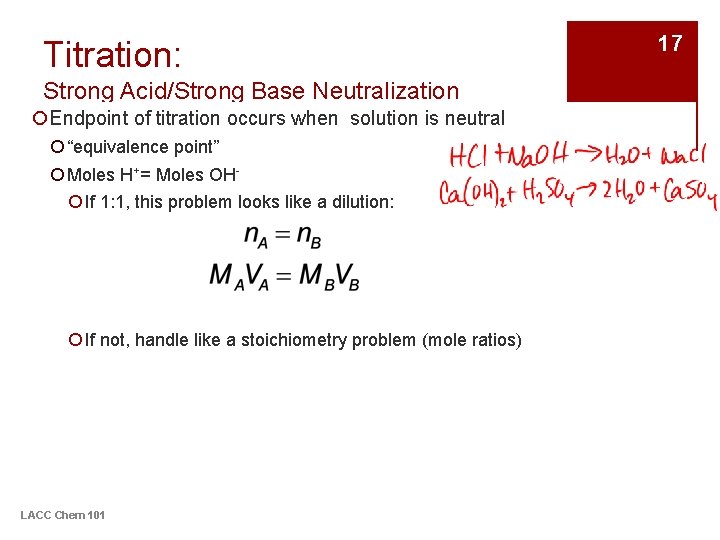
Titration: Strong Acid/Strong Base Neutralization ¡Endpoint of titration occurs when solution is neutral ¡ “equivalence point” ¡ Moles H+= Moles OH¡ If 1: 1, this problem looks like a dilution: ¡ If not, handle like a stoichiometry problem (mole ratios) LACC Chem 101 17

Acid-Base Titrations ¡Volumetric analysis of acids and bases ¡Solution used is called titrant ¡Buret measures volumes very precisely ¡Equivalence point is when both solutions react stoichiometrically with one another ¡ Endpoint of titration is indicated by a color indicator ¡ Several types available, depending on target p. H ¡ Most common is phenolphthalein ¡ Indicates near p. H 7 LACC Chem 101 18

Requirements for Acid/Base Titrations 19 1. The concentration of the titrant must be known ¡ called the standard solution 2. The exact reaction between the titrant and reacted substance must be known 3. The equivalence point must be known. ¡ An indicator that changes color at, or very near, the equivalence point is often used 4. The point at which the indicator changes color is called the end point. The goal is to choose an indicator whose end point coincides with the equivalence point. ¡ NOTE: Equivalence Point is NOT THE SAME as End Point! WHY? ? ? ¡ 5. The volume of titrant required to reach the equivalence point must be known (measured) as accurately as possible. LACC Chem 101
![p H of Solutions p H log H 3 O p H p. H of Solutions p. H = - log [H 3 O+] p. H](https://slidetodoc.com/presentation_image_h2/48cc0d6789519ed8c6cbeb6d411fbe36/image-20.jpg)
p. H of Solutions p. H = - log [H 3 O+] p. H > 7 is referred to as a base p. H < 7 is referred to as an acid ¡Can be read using a p. H meter or litmus (indicator) paper ¡ Measurements taken during or at the end of experiment ¡ p. H curve tells characteristics of acid ¡Titration of acid carried out with base, and titration of base carried out with acids ¡ p. H will change as a function of volume of standard used LACC Chem 101 20

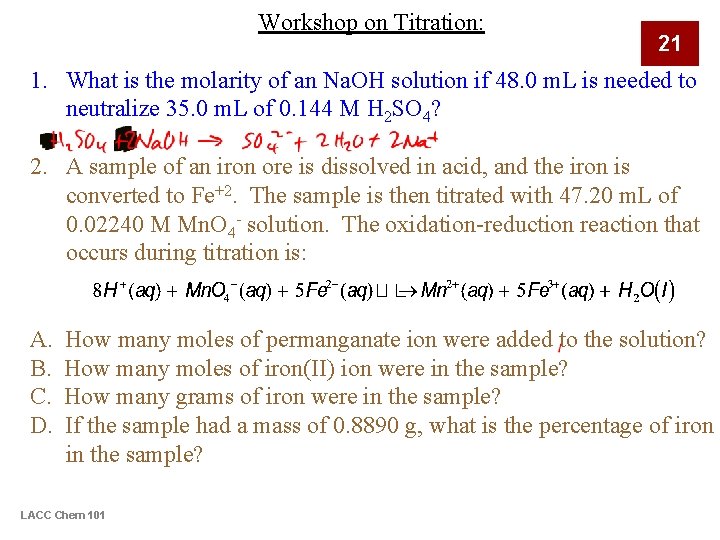
Workshop on Titration: 21 1. What is the molarity of an Na. OH solution if 48. 0 m. L is needed to neutralize 35. 0 m. L of 0. 144 M H 2 SO 4? 2. A sample of an iron ore is dissolved in acid, and the iron is converted to Fe+2. The sample is then titrated with 47. 20 m. L of 0. 02240 M Mn. O 4 - solution. The oxidation-reduction reaction that occurs during titration is: A. B. C. D. How many moles of permanganate ion were added to the solution? How many moles of iron(II) ion were in the sample? How many grams of iron were in the sample? If the sample had a mass of 0. 8890 g, what is the percentage of iron in the sample? LACC Chem 101