Introduction to Reactions Chemical Equation Reactants Products Fe





- Slides: 36

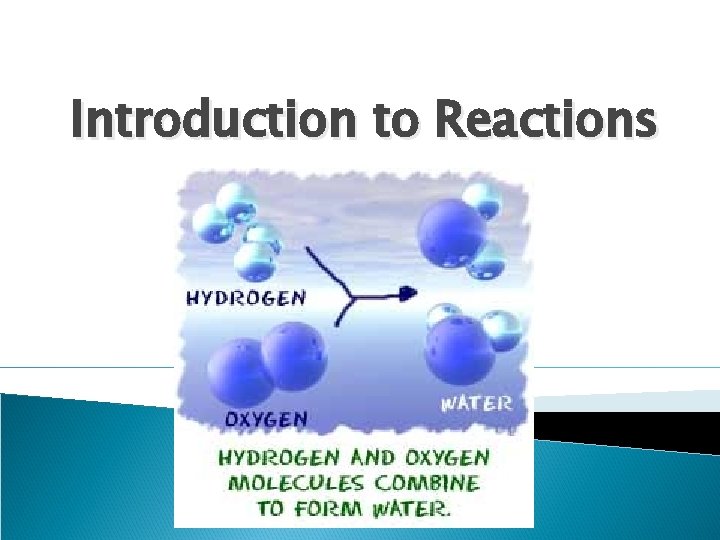
Introduction to Reactions

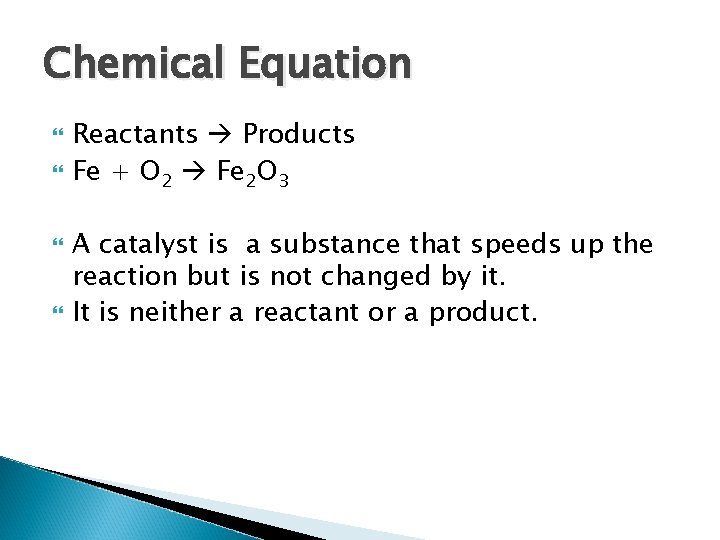
Chemical Equation Reactants Products Fe + O 2 Fe 2 O 3 A catalyst is a substance that speeds up the reaction but is not changed by it. It is neither a reactant or a product.

Signs of a Reaction Release of a gas ◦ CO 2 is released when acid is placed in a solution containing CO 32 - ions Formation of a solid (precipitate) ◦ A solution containing Ag+ ions mixed with a solution containing Cl- ions Heat is produced or absorbed ◦ Acid and base are mixed together Color changes

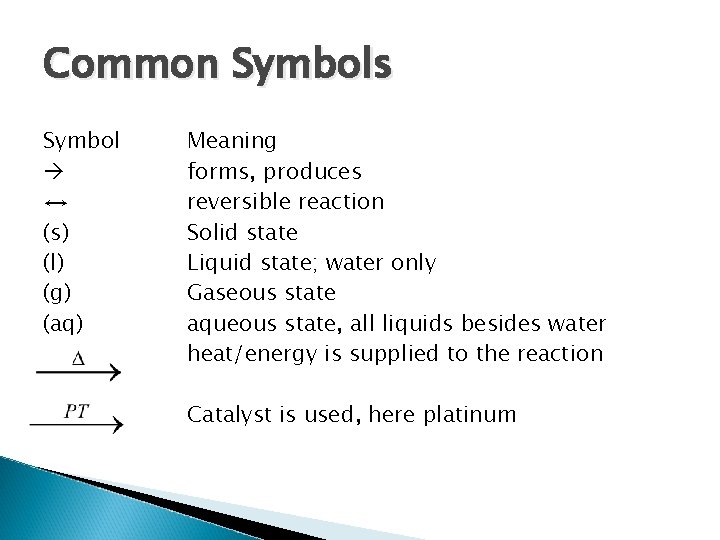
Common Symbols Symbol ↔ (s) (l) (g) (aq) Meaning forms, produces reversible reaction Solid state Liquid state; water only Gaseous state aqueous state, all liquids besides water heat/energy is supplied to the reaction Catalyst is used, here platinum

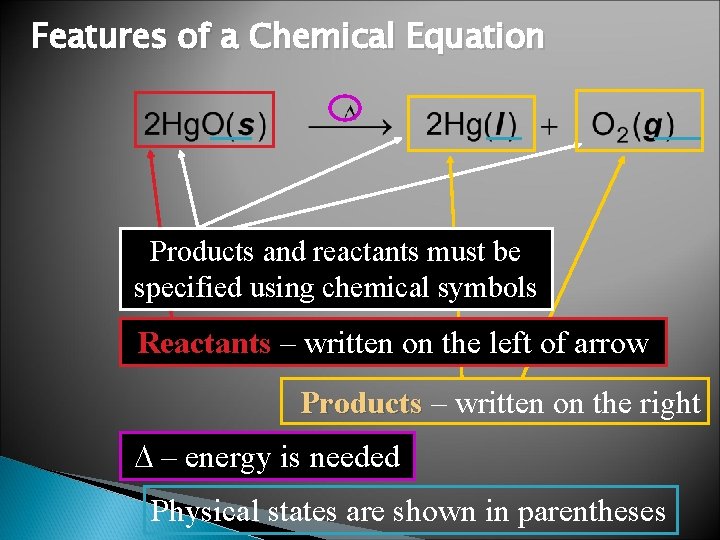
Features of a Chemical Equation Products and reactants must be specified using chemical symbols Reactants – written on the left of arrow Products – written on the right – energy is needed Physical states are shown in parentheses

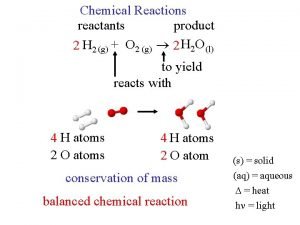
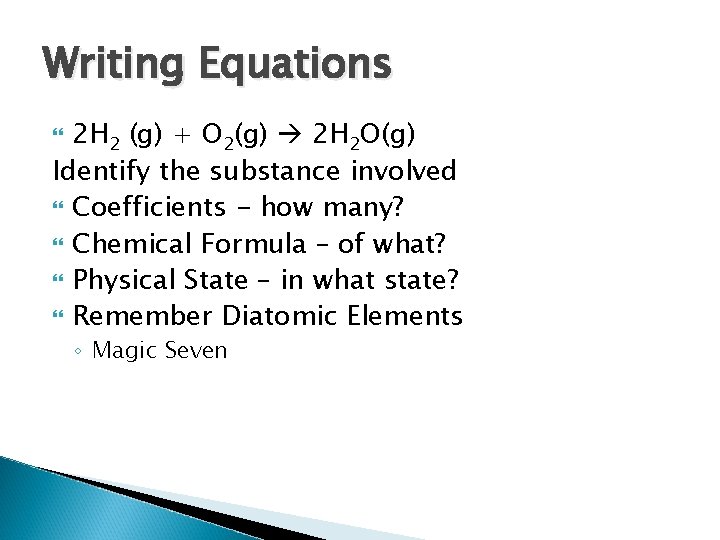
Writing Equations 2 H 2 (g) + O 2(g) 2 H 2 O(g) Identify the substance involved Coefficients - how many? Chemical Formula – of what? Physical State – in what state? Remember Diatomic Elements ◦ Magic Seven

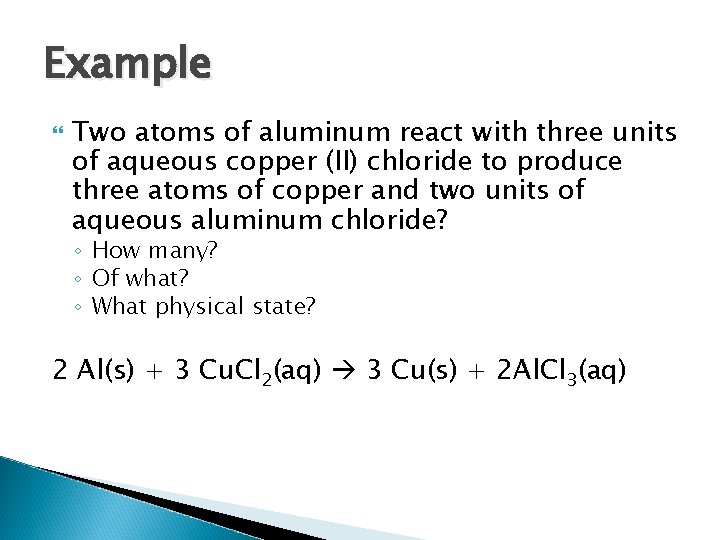
Example Two atoms of aluminum react with three units of aqueous copper (II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride? ◦ How many? ◦ Of what? ◦ What physical state?

Example Two atoms of aluminum react with three units of aqueous copper (II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride? ◦ How many? ◦ Of what? ◦ What physical state? 2 Al(s) + 3 Cu. Cl 2(aq) 3 Cu(s) + 2 Al. Cl 3(aq)

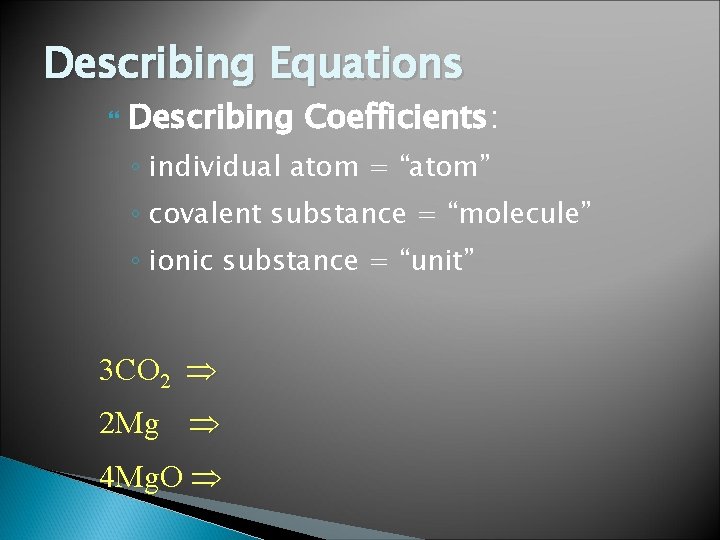
Describing Equations Describing Coefficients: ◦ individual atom = “atom” ◦ covalent substance = “molecule” ◦ ionic substance = “unit” 3 CO 2 2 Mg 4 Mg. O

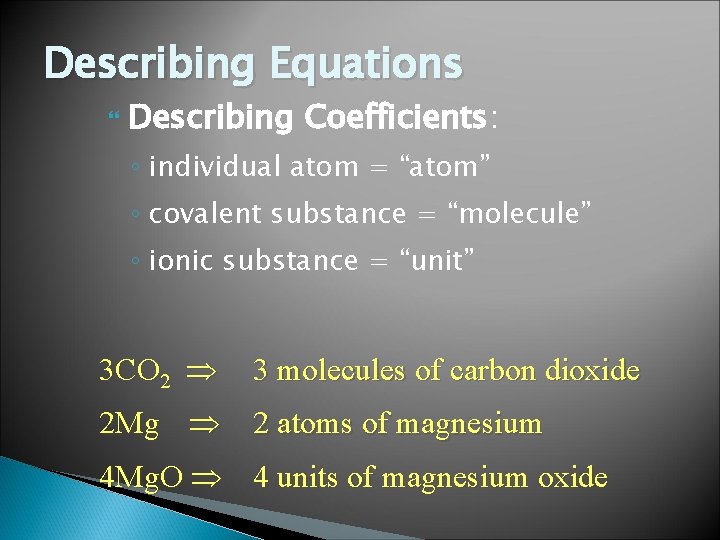
Describing Equations Describing Coefficients: ◦ individual atom = “atom” ◦ covalent substance = “molecule” ◦ ionic substance = “unit” 3 CO 2 3 molecules of carbon dioxide 2 Mg 2 atoms of magnesium 4 Mg. O 4 units of magnesium oxide

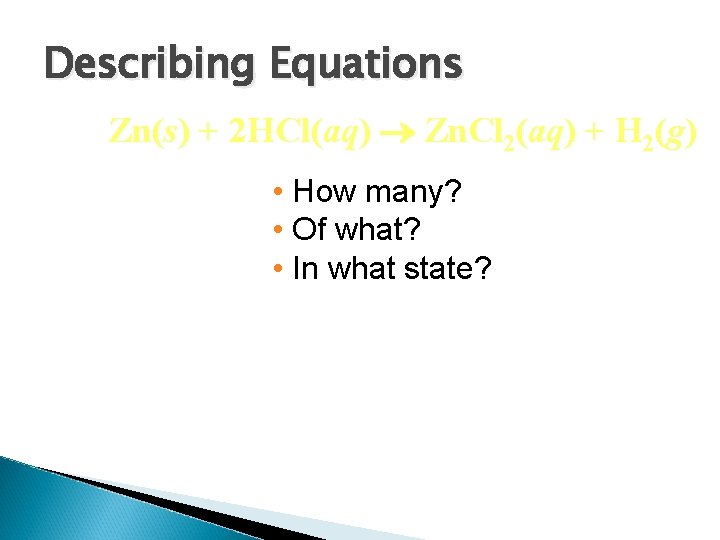
Describing Equations Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • How many? • Of what? • In what state?

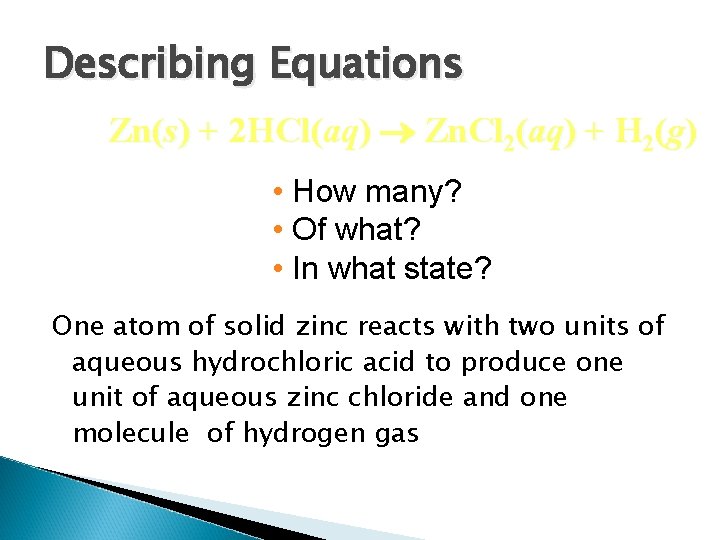
Describing Equations Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • How many? • Of what? • In what state? One atom of solid zinc reacts with two units of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas

Balancing Reaction

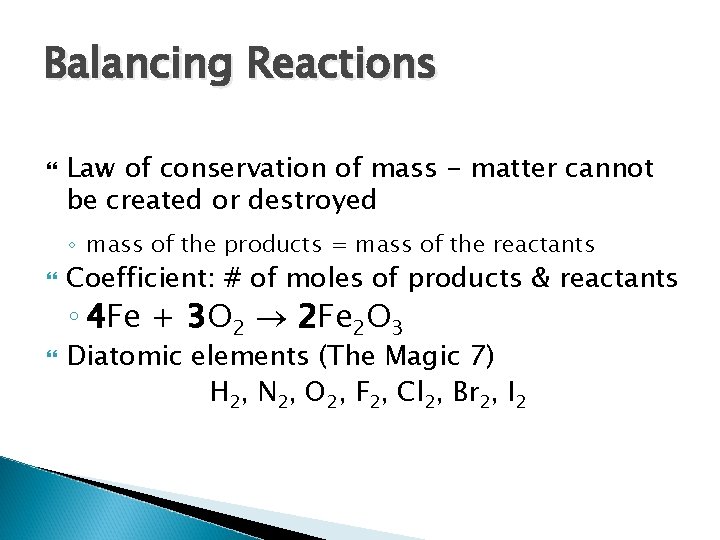
Balancing Reactions Law of conservation of mass - matter cannot be created or destroyed ◦ mass of the products = mass of the reactants Coefficient: # of moles of products & reactants ◦ 4 Fe + 3 O 2 2 Fe 2 O 3 Diatomic elements (The Magic 7) H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2

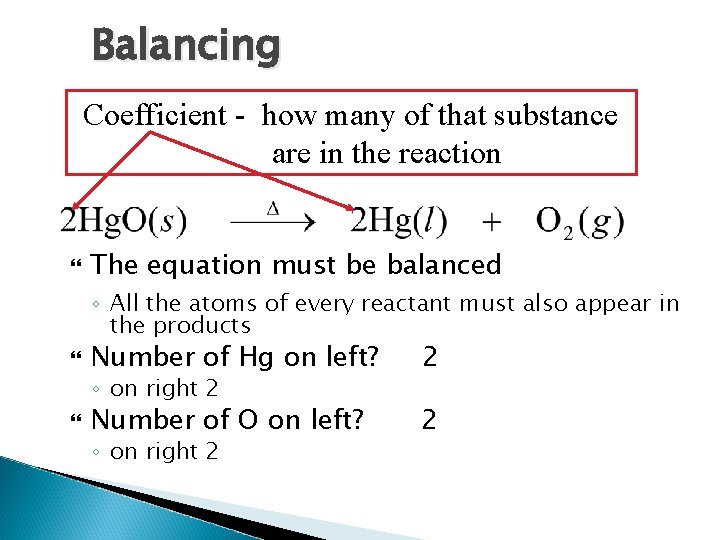
Balancing Coefficient - how many of that substance are in the reaction The equation must be balanced ◦ All the atoms of every reactant must also appear in the products Number of Hg on left? 2 Number of O on left? 2 ◦ on right 2

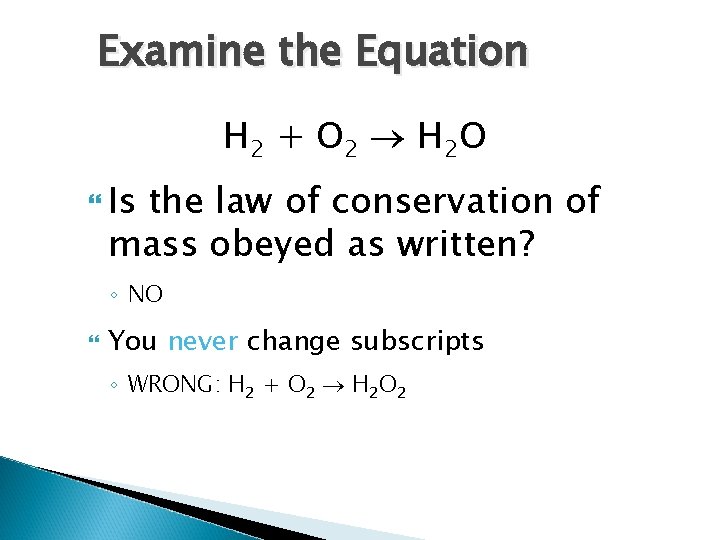
Examine the Equation H 2 + O 2 H 2 O Is the law of conservation of mass obeyed as written? ◦ NO You never change subscripts ◦ WRONG: H 2 + O 2 H 2 O 2

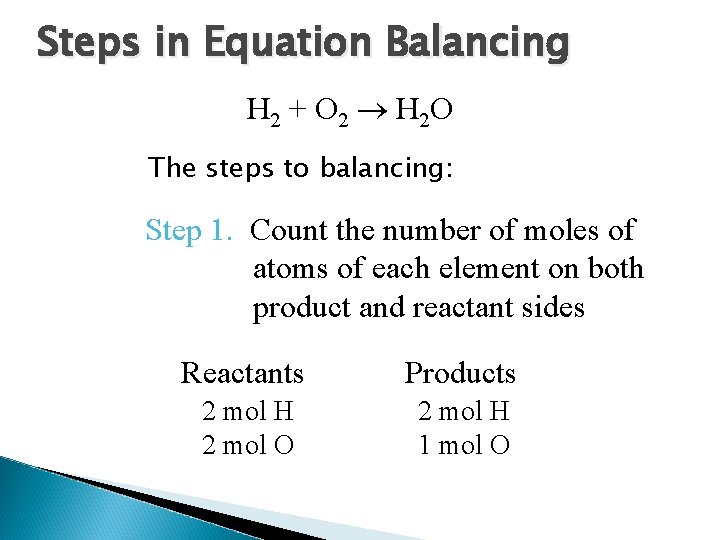
Steps in Equation Balancing H 2 + O 2 H 2 O The steps to balancing: Step 1. Count the number of moles of atoms of each element on both product and reactant sides Reactants Products 2 mol H 2 mol O 2 mol H 1 mol O

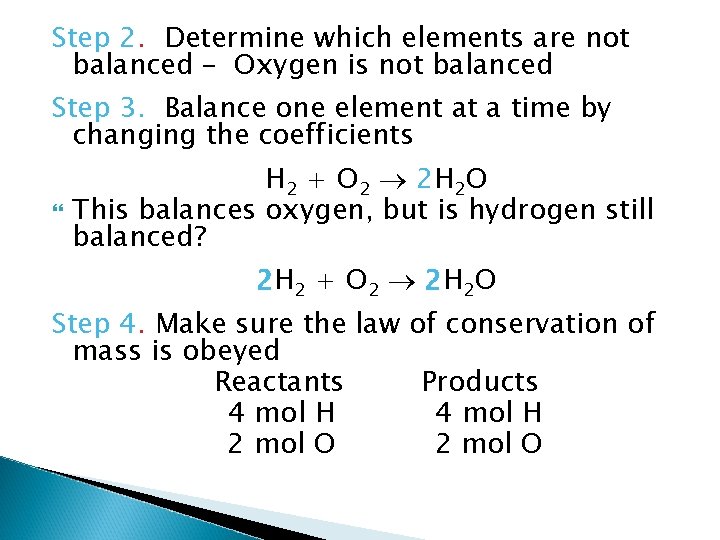
Step 2. Determine which elements are not balanced – Oxygen is not balanced Step 3. Balance one element at a time by changing the coefficients H 2 + O 2 2 H 2 O This balances oxygen, but is hydrogen still balanced? 2 H 2 + O 2 2 H 2 O Step 4. Make sure the law of conservation of mass is obeyed Reactants Products 4 mol H 2 mol O

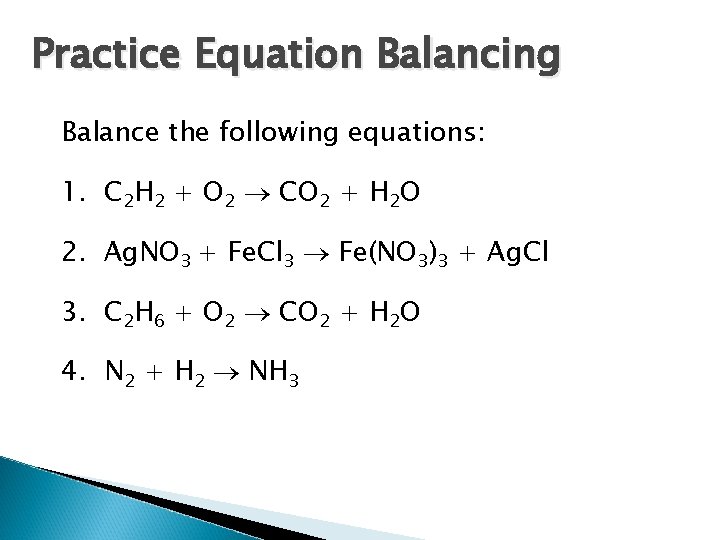
Practice Equation Balancing Balance the following equations: 1. C 2 H 2 + O 2 CO 2 + H 2 O 2. Ag. NO 3 + Fe. Cl 3 Fe(NO 3)3 + Ag. Cl 3. C 2 H 6 + O 2 CO 2 + H 2 O 4. N 2 + H 2 NH 3

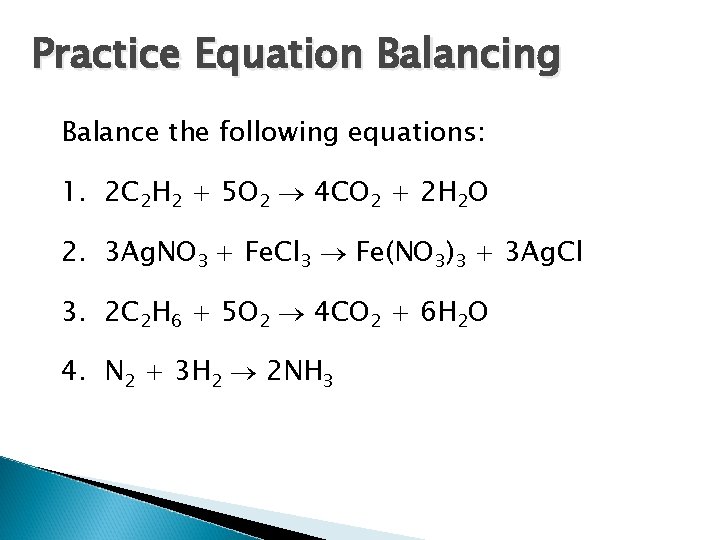
Practice Equation Balancing Balance the following equations: 1. 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O 2. 3 Ag. NO 3 + Fe. Cl 3 Fe(NO 3)3 + 3 Ag. Cl 3. 2 C 2 H 6 + 5 O 2 4 CO 2 + 6 H 2 O 4. N 2 + 3 H 2 2 NH 3

Types of Reaction

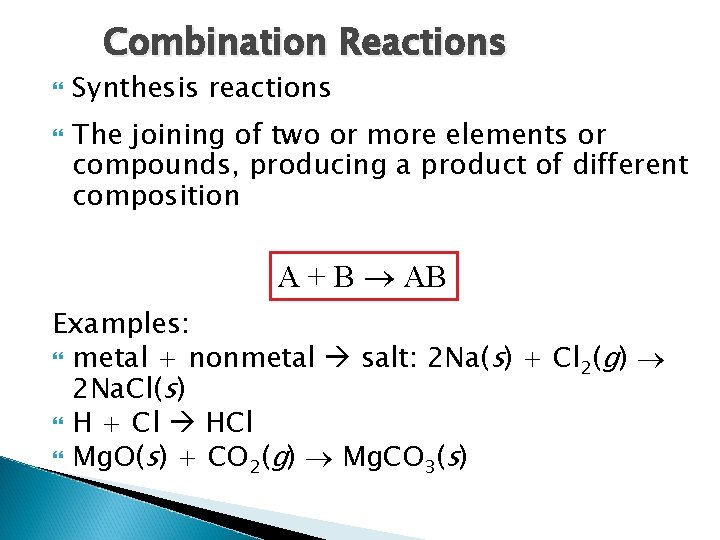
Combination Reactions Synthesis reactions The joining of two or more elements or compounds, producing a product of different composition A + B AB Examples: metal + nonmetal salt: 2 Na(s) + Cl 2(g) 2 Na. Cl(s) H + Cl HCl Mg. O(s) + CO 2(g) Mg. CO 3(s)

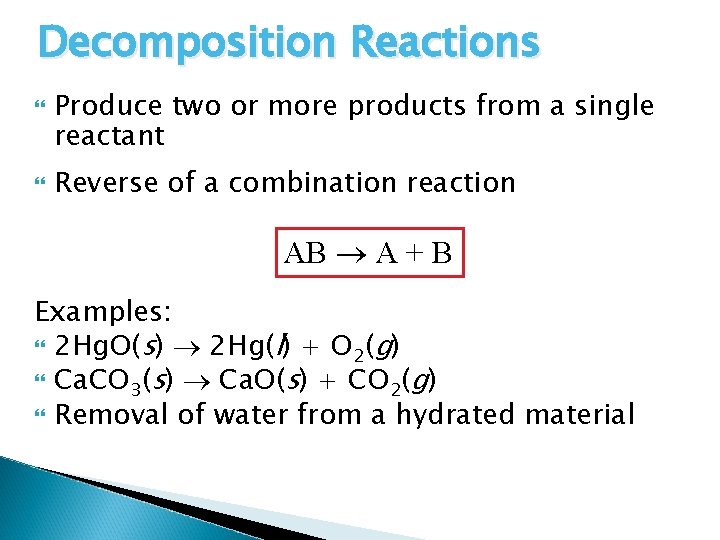
Decomposition Reactions Produce two or more products from a single reactant Reverse of a combination reaction AB A + B Examples: 2 Hg. O(s) 2 Hg(l) + O 2(g) Ca. CO 3(s) Ca. O(s) + CO 2(g) Removal of water from a hydrated material

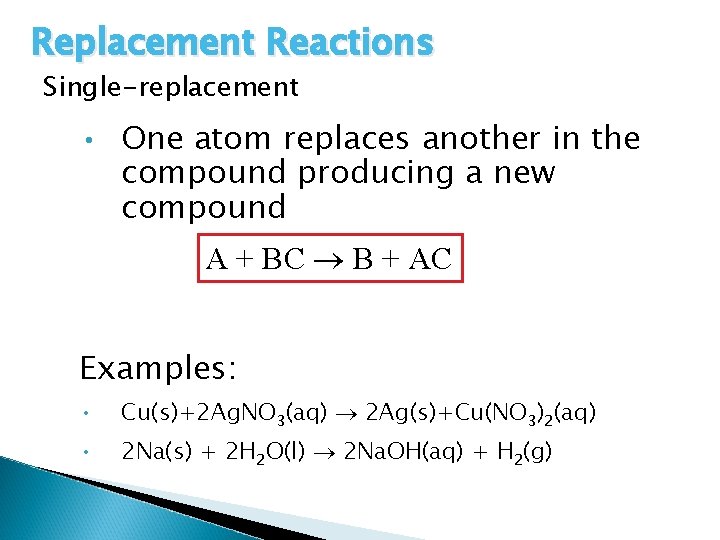
Replacement Reactions Single-replacement • One atom replaces another in the compound producing a new compound A + BC B + AC Examples: • Cu(s)+2 Ag. NO 3(aq) 2 Ag(s)+Cu(NO 3)2(aq) • 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g)

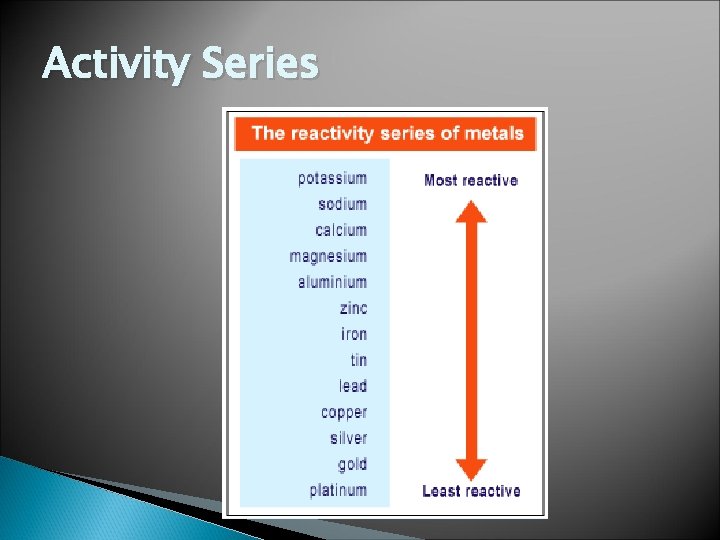
Single Replacement Rxn. Activity Series – lists metals in order of decreasing reactivity (p. 333) Reactive metals will replace any metal listed below it in the activity series If the metal is below, no reaction occurs Halogen(7 A) can replace other halogens that are below it in the periodic table

Activity Series

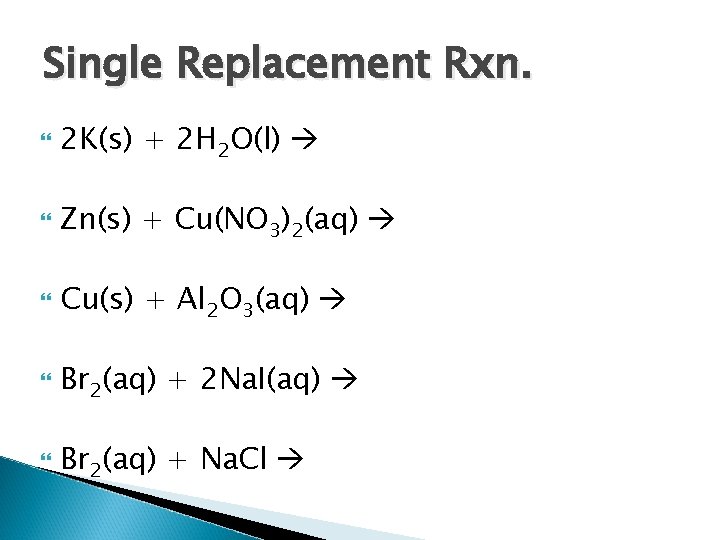
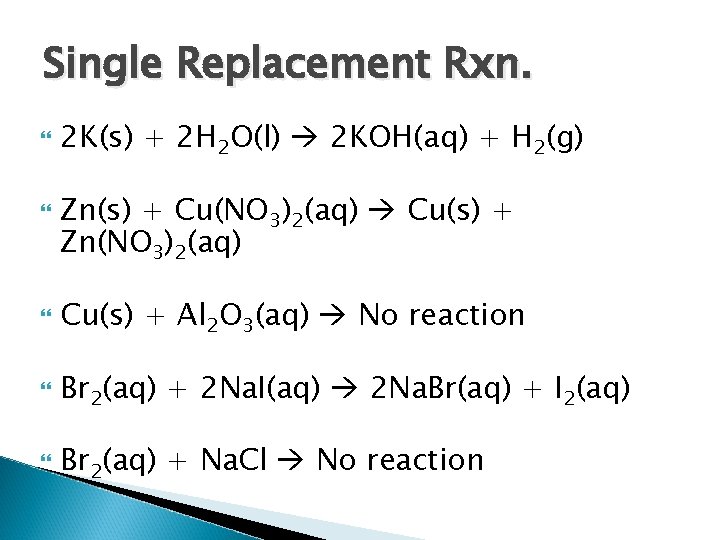
Single Replacement Rxn. 2 K(s) + 2 H 2 O(l) Zn(s) + Cu(NO 3)2(aq) Cu(s) + Al 2 O 3(aq) Br 2(aq) + 2 Na. I(aq) Br 2(aq) + Na. Cl

Single Replacement Rxn. 2 K(s) + 2 H 2 O(l) 2 KOH(aq) + H 2(g) Zn(s) + Cu(NO 3)2(aq) Cu(s) + Zn(NO 3)2(aq) Cu(s) + Al 2 O 3(aq) No reaction Br 2(aq) + 2 Na. I(aq) 2 Na. Br(aq) + I 2(aq) Br 2(aq) + Na. Cl No reaction

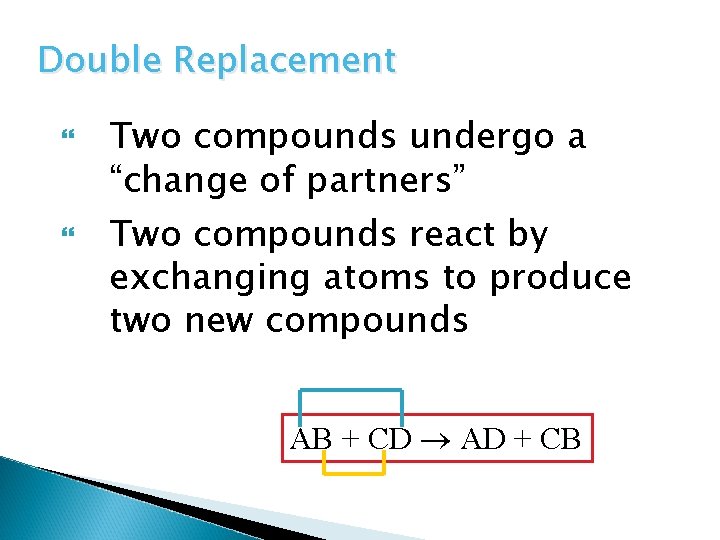
Double Replacement Two compounds undergo a “change of partners” Two compounds react by exchanging atoms to produce two new compounds AB + CD AD + CB

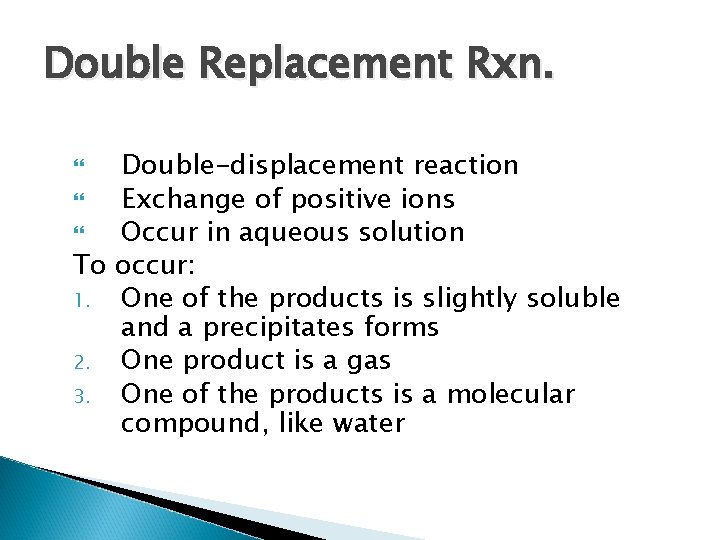
Double Replacement Rxn. Double-displacement reaction Exchange of positive ions Occur in aqueous solution To occur: 1. One of the products is slightly soluble and a precipitates forms 2. One product is a gas 3. One of the products is a molecular compound, like water

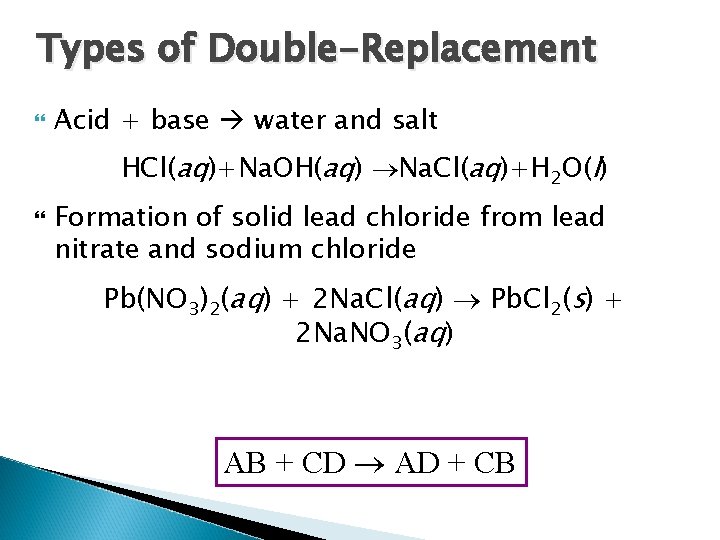
Types of Double-Replacement Acid + base water and salt HCl(aq)+Na. OH(aq) Na. Cl(aq)+H 2 O(l) Formation of solid lead chloride from lead nitrate and sodium chloride Pb(NO 3)2(aq) + 2 Na. Cl(aq) Pb. Cl 2(s) + 2 Na. NO 3(aq) AB + CD AD + CB

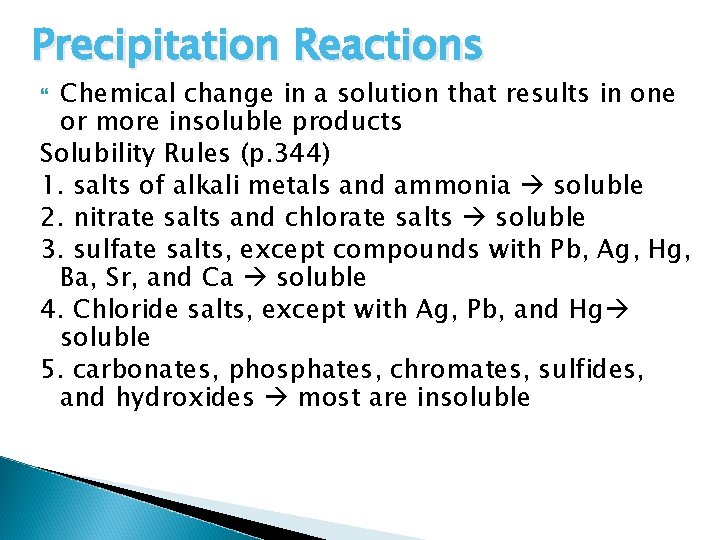
Precipitation Reactions Chemical change in a solution that results in one or more insoluble products Solubility Rules (p. 344) 1. salts of alkali metals and ammonia soluble 2. nitrate salts and chlorate salts soluble 3. sulfate salts, except compounds with Pb, Ag, Hg, Ba, Sr, and Ca soluble 4. Chloride salts, except with Ag, Pb, and Hg soluble 5. carbonates, phosphates, chromates, sulfides, and hydroxides most are insoluble

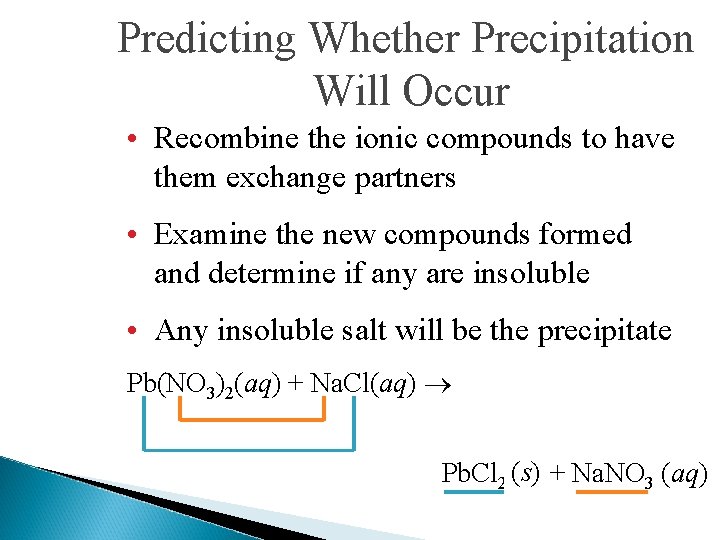
Predicting Whether Precipitation Will Occur • Recombine the ionic compounds to have them exchange partners • Examine the new compounds formed and determine if any are insoluble • Any insoluble salt will be the precipitate Pb(NO 3)2(aq) + Na. Cl(aq) Pb. Cl 2 (s) (? ) + Na. NO 3 ((aq) ? )

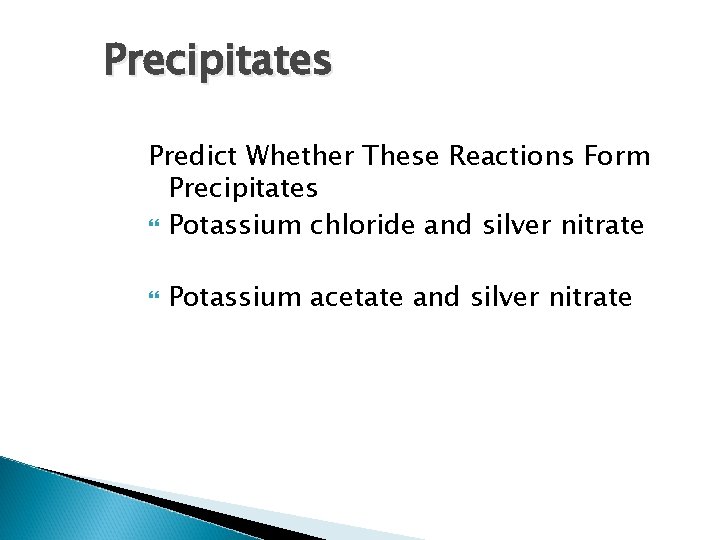
Precipitates Predict Whether These Reactions Form Precipitates Potassium chloride and silver nitrate Potassium acetate and silver nitrate

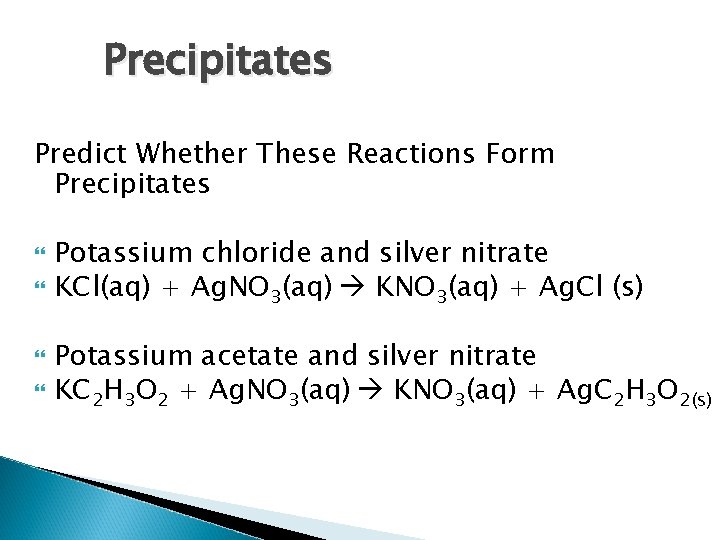
Precipitates Predict Whether These Reactions Form Precipitates Potassium chloride and silver nitrate KCl(aq) + Ag. NO 3(aq) KNO 3(aq) + Ag. Cl (s) Potassium acetate and silver nitrate KC 2 H 3 O 2 + Ag. NO 3(aq) KNO 3(aq) + Ag. C 2 H 3 O 2(s)

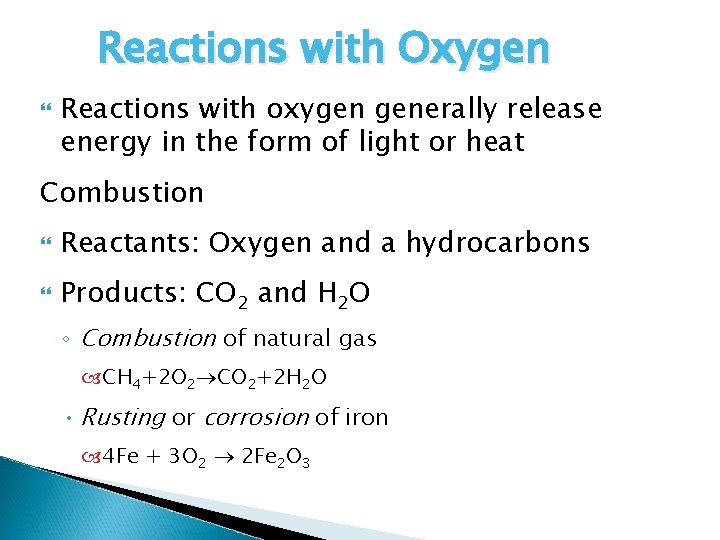
Reactions with Oxygen Reactions with oxygen generally release energy in the form of light or heat Combustion Reactants: Oxygen and a hydrocarbons Products: CO 2 and H 2 O ◦ Combustion of natural gas CH 4+2 O 2 CO 2+2 H 2 O • Rusting or corrosion of iron 4 Fe + 3 O 2 2 Fe 2 O 3
 Reactants and products
Reactants and products Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Synthesis reaction predicting products
Synthesis reaction predicting products Activity series of metals
Activity series of metals Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
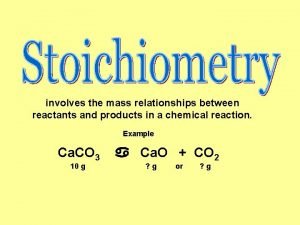
Chapter 18 chemical reactions balancing chemical equations Stoichiometry
Stoichiometry Phet products reactants and leftovers
Phet products reactants and leftovers Enthalpy of products
Enthalpy of products Reaction type: synthesis
Reaction type: synthesis What are the reactants and products of photosynthesis
What are the reactants and products of photosynthesis Activity 2 limiting reactants activity
Activity 2 limiting reactants activity Elephant toothpaste reactants and products
Elephant toothpaste reactants and products Reactants and products
Reactants and products Reactants products and leftovers
Reactants products and leftovers Reactants minus products bond energies
Reactants minus products bond energies Products and reactants
Products and reactants Relationship between reactants and products
Relationship between reactants and products Enthalpy graph
Enthalpy graph 20 examples of redox reaction
20 examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Predict the products of the following reactions.
Predict the products of the following reactions. Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Stoichiometry island diagram
Stoichiometry island diagram Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Balancing redox reactions
Balancing redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions How to identify type of reaction
How to identify type of reaction 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions