Introduction to Pulmonary Artery Denervation Rationale Technology and













- Slides: 27

Introduction to Pulmonary Artery Denervation: Rationale, Technology, and Clinical Update Ori Ben-Yehuda, MD, FACC University of California, San Diego & Cardiovascular Research Foundation New York, NY

Disclosures • I, Ori Ben-Yehuda, have no financial COI in regards to PAH medications/devices • Member of SAB for Sonivie, Inc

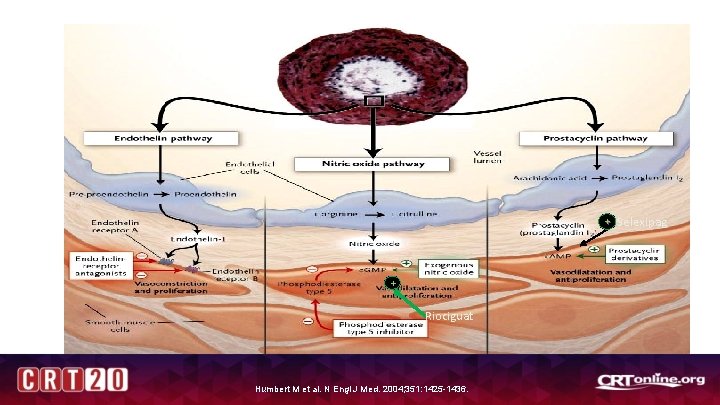
PAH Specific Therapy + Selexipag + Riociguat Humbert M et al. N Engl J Med. 2004; 351: 1425 -1436.

Challenges in Drug Therapy Development • Animal models not fully predictive (eg, monocrotaline mouse model – lots of drugs show efficacy- eg simvastatin; sugen-hypoxia model- ASK-1) • Anecdotal experiences in small number of patients may be type I error (example: ciclatinin) • Side effect limitations (eg: imatinib-Gleevec) • Patent Issues • $$$

ASK-1 Inhibition: From promising preclinical to failed phase II • • Pre-Clinical Budas et al. Am J Respir Crit Care Med Vol 197, pp 373 -385. – – – Apoptosis signal regulating kinase 1 Mitogen-activated protein kinase (MAP 3 K) Oxidative Stress activated kinases stimulate apoptosis, inflammation, and fibroiss • Clinical – – – ARROW trial Selosertib (2, 6, 18 mg daily) N=150 including placebo group PAH patients; 92% on combination Rx 24 week therapy No significant change in PVR, 6 MWD, FC, NTpro. BNP Presented at ERS 2017

Pulmonary Artery Denervation

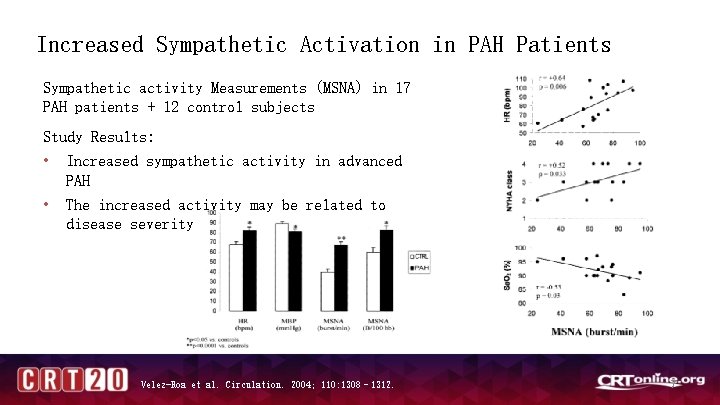
Increased Sympathetic Activation in PAH Patients Sympathetic activity Measurements (MSNA) in 17 PAH patients + 12 control subjects Study Results: • Increased sympathetic activity in advanced PAH • The increased activity may be related to disease severity Velez-Roa et al. Circulation. 2004; 110: 1308– 1312. 7

Prognostic Significance of Sympathetic Activation in PAH Patients Correlation between sympathetic activity and clinical condition in 32 PAH patients Study Results: • Sympathetic activation is a predictor of severe deterioration in patients with PAH Ciarka A et al. Am J Respir Crit Care Med 2010


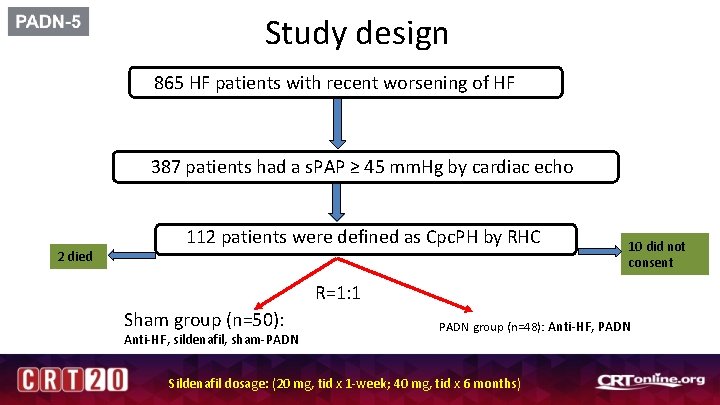
Study design 865 HF patients with recent worsening of HF 387 patients had a s. PAP ≥ 45 mm. Hg by cardiac echo 2 died 112 patients were defined as Cpc. PH by RHC 10 did not consent R=1: 1 Sham group (n=50): Anti-HF, sildenafil, sham-PADN group (n=48): Anti-HF, PADN Sildenafil dosage: (20 mg, tid x 1 -week; 40 mg, tid x 6 months)

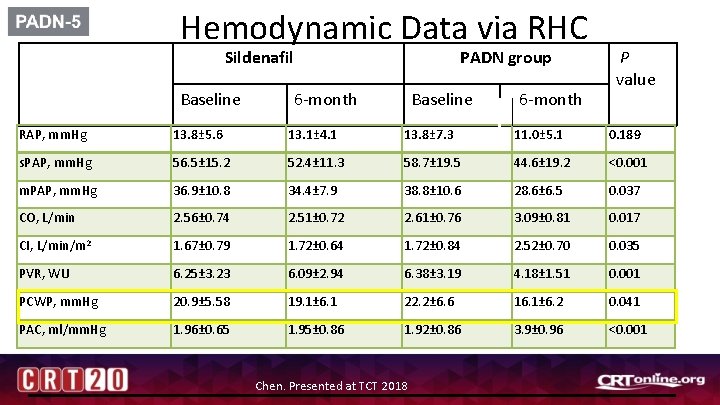
Hemodynamic Data via RHC Sildenafil Baseline PADN group 6 -month Baseline 6 -month P value RAP, mm. Hg 13. 8± 5. 6 13. 1± 4. 1 13. 8± 7. 3 11. 0± 5. 1 0. 189 s. PAP, mm. Hg 56. 5± 15. 2 52. 4± 11. 3 58. 7± 19. 5 44. 6± 19. 2 <0. 001 m. PAP, mm. Hg 36. 9± 10. 8 34. 4± 7. 9 38. 8± 10. 6 28. 6± 6. 5 0. 037 CO, L/min 2. 56± 0. 74 2. 51± 0. 72 2. 61± 0. 76 3. 09± 0. 81 0. 017 CI, L/min/m 2 1. 67± 0. 79 1. 72± 0. 64 1. 72± 0. 84 2. 52± 0. 70 0. 035 PVR, WU 6. 25± 3. 23 6. 09± 2. 94 6. 38± 3. 19 4. 18± 1. 51 0. 001 PCWP, mm. Hg 20. 9± 5. 58 19. 1± 6. 1 22. 2± 6. 6 16. 1± 6. 2 0. 041 PAC, ml/mm. Hg 1. 96± 0. 65 1. 95± 0. 86 1. 92± 0. 86 3. 9± 0. 96 <0. 001 Chen. Presented at TCT 2018

Soni. Vie Technology - TIVUS™ System Non-Focused Ultrasound for intra-vascular denervation The system and the catheter obtained CE mark for renal denervation application

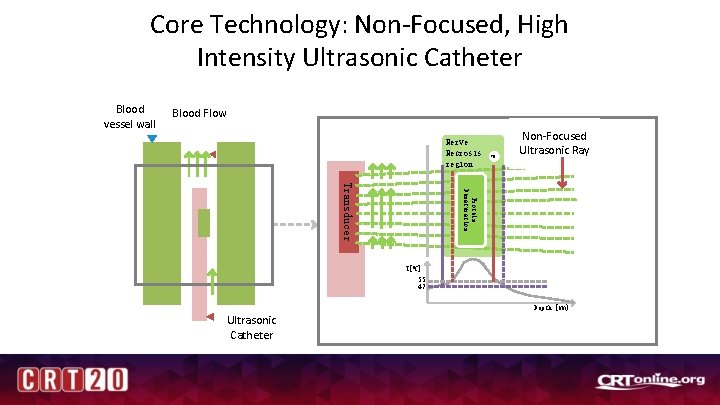
Core Technology: Non-Focused, High Intensity Ultrasonic Catheter Blood vessel wall Blood Flow Nerve Necrosis region XN Non-Focused Ultrasonic Ray Protein Denaturation Transducer T[0 C] 55 47 Depth [mm} Ultrasonic Catheter

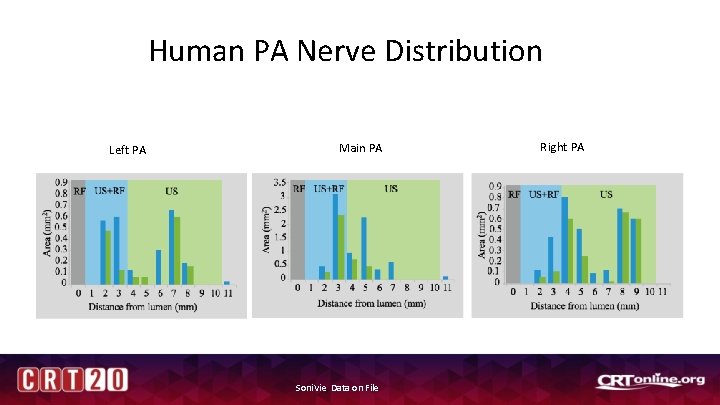
Human PA Nerve Distribution

Human PA Nerve Distribution Left PA Main PA Soni. Vie, Data on File Right PA

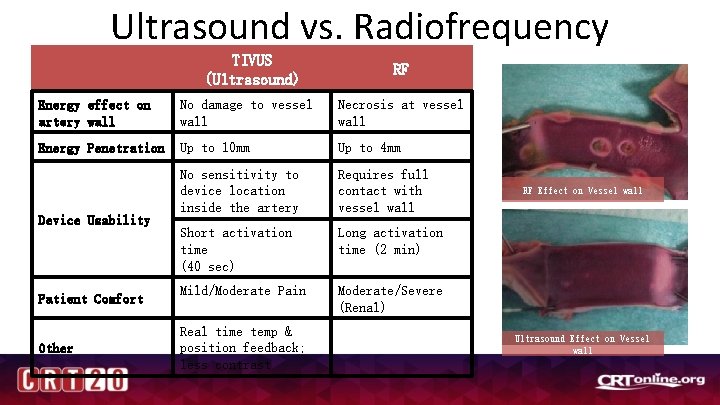
Ultrasound vs. Radiofrequency Ablation TIVUS (Ultrasound) RF Energy effect on artery wall No damage to vessel wall Necrosis at vessel wall Energy Penetration Up to 10 mm Up to 4 mm No sensitivity to device location inside the artery Requires full contact with vessel wall Short activation time (40 sec) Long activation time (2 min) Mild/Moderate Pain Moderate/Severe (Renal) Device Usability Patient Comfort Other Real time temp & position feedback; less contrast RF Effect on Vessel wall Ultrasound Effect on Vessel wall

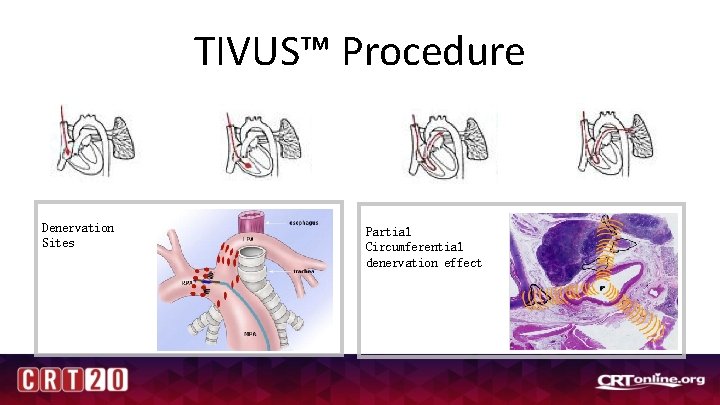
TIVUS™ Procedure RHC - Right Heart catheterization Denervation Sites Partial Circumferential denervation effect

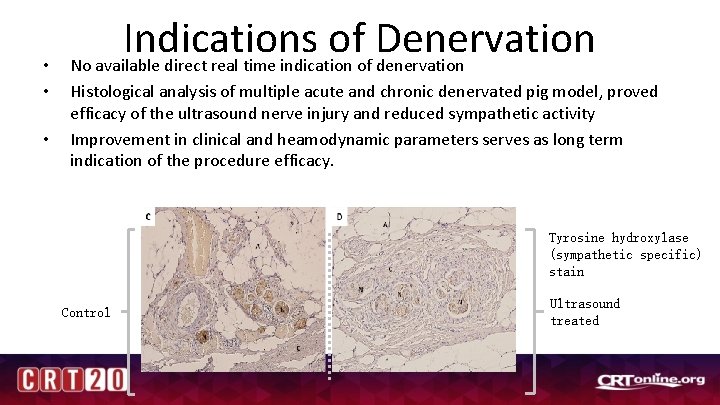
• • • Indications of Denervation No available direct real time indication of denervation Histological analysis of multiple acute and chronic denervated pig model, proved efficacy of the ultrasound nerve injury and reduced sympathetic activity Improvement in clinical and heamodynamic parameters serves as long term indication of the procedure efficacy. Tyrosine hydroxylase (sympathetic specific) stain Control Ultrasound treated

Pulmonary Artery Denervation

European/Israel and US Studies: Demographics Number of patients Age, years mean (SD) Gender, n (% female) EU Patients 13 63 (10. 8) 11 (85%) US Patients 9 55. 5 (12. 2) 5 (56%) Race, n (%) African Caucasian Other 1 (10%) 12 (92. 3%) - 0 6 (67%) 3 (33%) Time from Diagnosis of PAH, years mean (SD) 6. 1 (6. 4) 5. 7 (5. 7) Type of PAH, n (%) Connective tissue Disease Drug induced Idiopathic PAH 7 (53. 8%) 3 (23. 1%) 4 (44%) 0 5 (56%) PAH therapy, n (%) ERA and PDE 5 7 (58. 3%) 3 (33%) ERA and s. GC and inhaled Prostacyclin ERA and PDE 5 and inhaled Prostacyclin Diuretics therapy, n (%) 4 (33. 3%) 0 1 (8. 3%) 9 (75%) 0 3 (33%) 7 (78%)

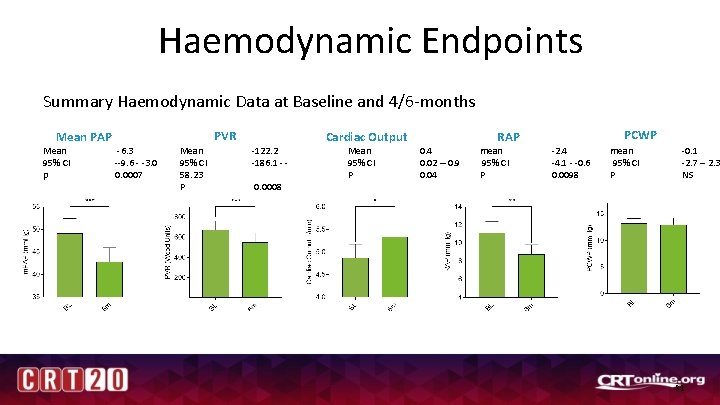
Haemodynamic Endpoints Summary Haemodynamic Data at Baseline and 4/6 -months PVR Mean PAP Mean 95% CI p -6. 3 --9. 6 - -3. 0 0. 0007 Mean 95% CI 58. 23 P -122. 2 -186. 1 - 0. 0008 Cardiac Output Mean 95% CI P PCWP RAP 0. 4 0. 02 – 0. 9 0. 04 mean 95% CI P -2. 4 -4. 1 - -0. 6 0. 0098 mean 95% CI P -0. 1 -2. 7 – 2. 3 NS 21

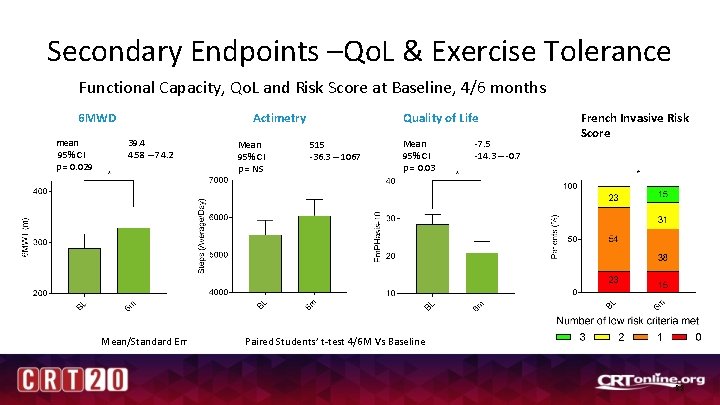
Secondary Endpoints –Qo. L & Exercise Tolerance Functional Capacity, Qo. L and Risk Score at Baseline, 4/6 months Actimetry 6 MWD mean 95% CI p = 0. 029 39. 4 4. 58 – 74. 2 Mean/Standard Err Mean 95% CI p = NS Quality of Life 515 -36. 3 – 1067 Mean 95% CI p = 0. 03 -7. 5 -14. 3 – -0. 7 French Invasive Risk Score Paired Students’ t-test 4/6 M Vs Baseline 22

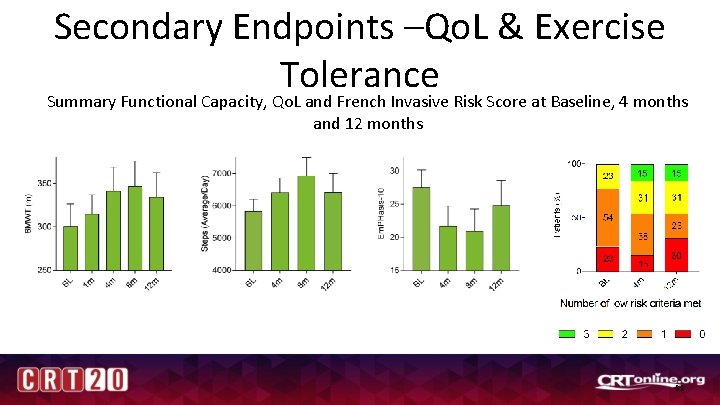
Secondary Endpoints –Qo. L & Exercise Tolerance Summary Functional Capacity, Qo. L and French Invasive Risk Score at Baseline, 4 months and 12 months mean ± sd = -6. 9 ± 14 p = 0. 275 % Change = -21 % mean ± sd = 45. 5± 64. 6 p = 0. 065 % Change = 21 % mean ± sd = 584± 883 p = 0. 141 % Change = 12 % 23

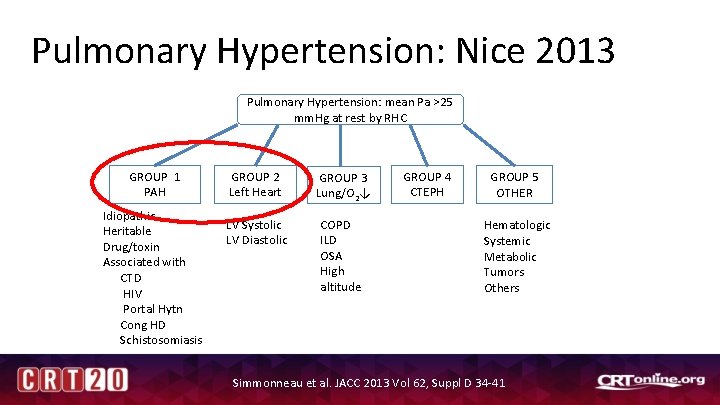
Pulmonary Hypertension: Nice 2013 Pulmonary Hypertension: mean Pa >25 mm. Hg at rest by RHC GROUP 1 PAH Idiopathic Heritable Drug/toxin Associated with CTD HIV Portal Hytn Cong HD Schistosomiasis GROUP 2 Left Heart GROUP 3 Lung/O 2↓ LV Systolic LV Diastolic COPD ILD OSA High altitude GROUP 4 CTEPH GROUP 5 OTHER Hematologic Systemic Metabolic Tumors Others Simmonneau et al. JACC 2013 Vol 62, Suppl D 34 -41

TROPHY-2: Study Objectives • The objective of this study is to assess the safety and initial effectiveness of the TIVUS™ System when used for pulmonary artery denervation in group II PH patients through change in clinical parameters including hemodynamics, exercise tolerance, and quality of life.

TROPHY-II Primary Outcome Measure (Safety): Procedural related Adverse Events (complications) at up to 30 days post procedure including pulmonary artery perforation/dissection, acute thrombus formation in the pulmonary artery, pulmonary artery aneurysm, vascular stenosis, hemoptysis, all cause PH related as well as procedural related death.

Secondary Outcome Measure (Clinical effectiveness)- at 4 months • • • Changes in cardiopulmonary exercise test (CPET) results (Peak VO 2, VE/VCO 2 slope, VO 2 at anaerobic threshold, peak workload) from baseline Change in resting and exercise hemodynamic parameters including mean right atrial pressure (m. RAP), mean pulmonary artery pressure (m. PAP), pulmonary vascular resistance (PVR), systemic vascular resistance (SVR) and cardiac index (CI) from baseline Changes in 6 MWD from baseline Changes in Echocardiography parameters including Right Ventricular function from baseline Change in NT-BNP levels from baseline