Introduction to PCR and q PCR Part II






















- Slides: 53

Introduction to PCR and q. PCR Part II: PCR!! Dr. Chaim Wachtel

q. PCR technical workflow DNA Extraction Sampling q. PCR RNA Extraction 1. Different methods 2. Importance of high quality RNA DNase treatment Data Analysis Reverse Transcription 1. Choice of primer 2. Choice of enzyme 3. Variability of reaction

Primer design

Primer design – key to successful PCR • Good primer design saves time and money • Advanced applications require even more stringent primer design – Multiplex – Low abundance

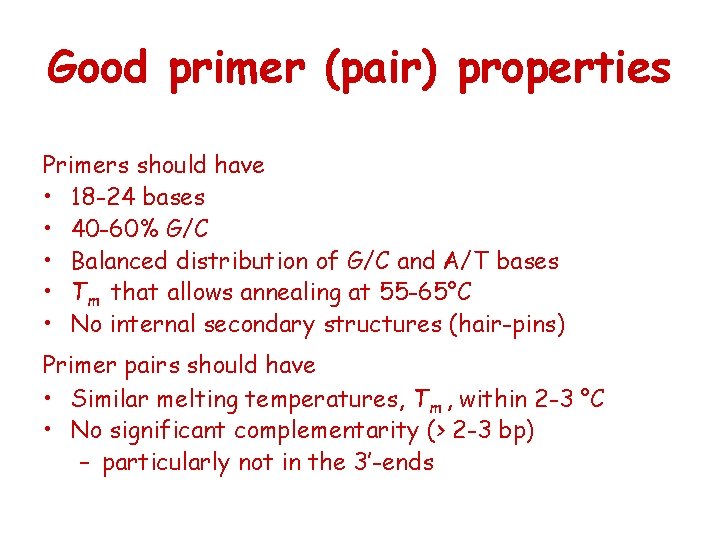
Good primer (pair) properties Primers should have • 18 -24 bases • 40 -60% G/C • Balanced distribution of G/C and A/T bases • Tm that allows annealing at 55 -65°C • No internal secondary structures (hair-pins) Primer pairs should have • Similar melting temperatures, Tm , within 2 -3 °C • No significant complementarity (> 2 -3 bp) – particularly not in the 3’-ends

The primer dimer (PD) problem • Primers that interact are amplified by PCR. • PD formation competes with the designed PCR and can compromise the reaction efficiency. Sense ´ 3 Antisense Cycling. . . Sense c. Antisense Sense Antisense

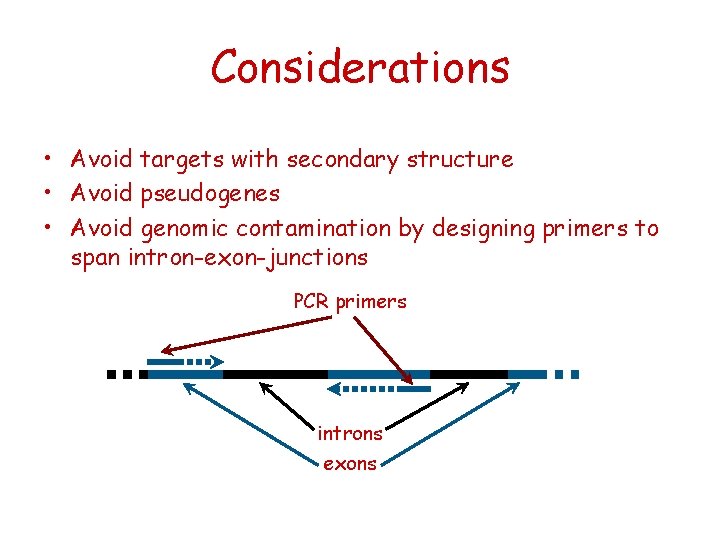
Considerations • Avoid targets with secondary structure • Avoid pseudogenes • Avoid genomic contamination by designing primers to span intron-exon-junctions PCR primers introns exons

Links for designing primers • http: //www. tataa. com/ • http: //www. ncbi. nlm. nih. gov/BLAST/ • www. premierbiosoft. com/netprimer/netprlau nch/netprlaunch. html • www. ensembl. org • http: //www-genome. wi. mit. edu/cgibin/primer 3_www. cgi • http: //www. bioinfo. rpi. edu/applications/mfo ld/dna/form 1. cgi • Primer Design- Beacon Designer/Alelle. ID • Primer express 3 (AB)

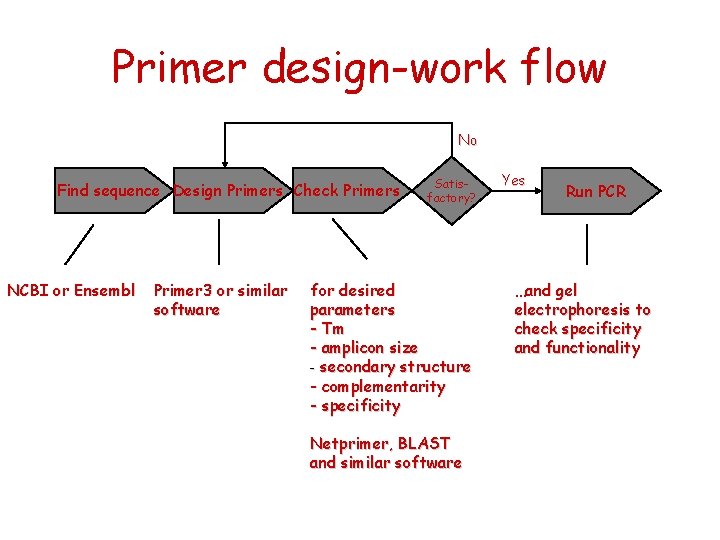
Primer design-work flow No Find sequence Design Primers Check Primers NCBI or Ensembl Primer 3 or similar software Satisfactory? for desired parameters - Tm - amplicon size - secondary structure - complementarity - specificity Netprimer, BLAST and similar software Yes Run PCR …and gel electrophoresis to check specificity and functionality

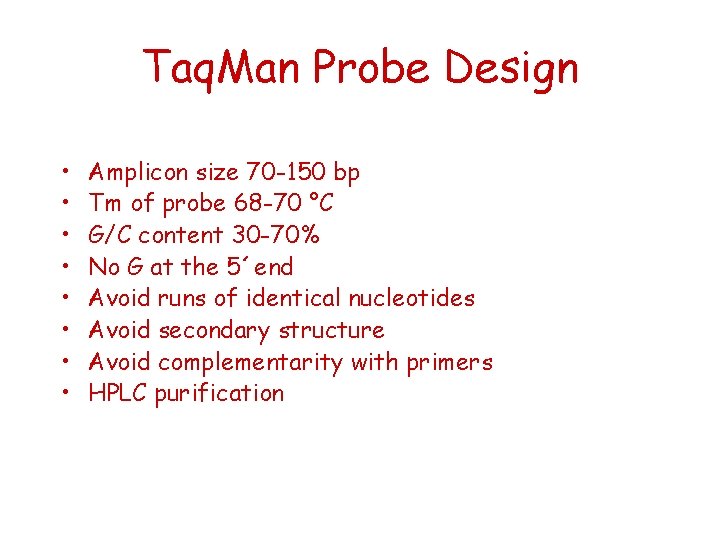
Taq. Man Probe Design • • Amplicon size 70 -150 bp Tm of probe 68 -70 °C G/C content 30 -70% No G at the 5´end Avoid runs of identical nucleotides Avoid secondary structure Avoid complementarity with primers HPLC purification

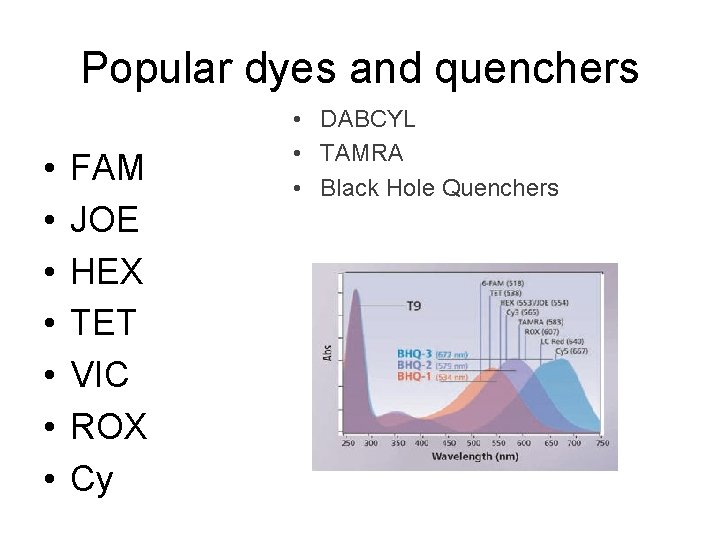
Popular dyes and quenchers • • FAM JOE HEX TET VIC ROX Cy • DABCYL • TAMRA • Black Hole Quenchers

RT-PCR • Housekeeping genes – What are they – How do you choose • • • Standard curve Primer Dimer Melt curve Optimization Test samples Reference

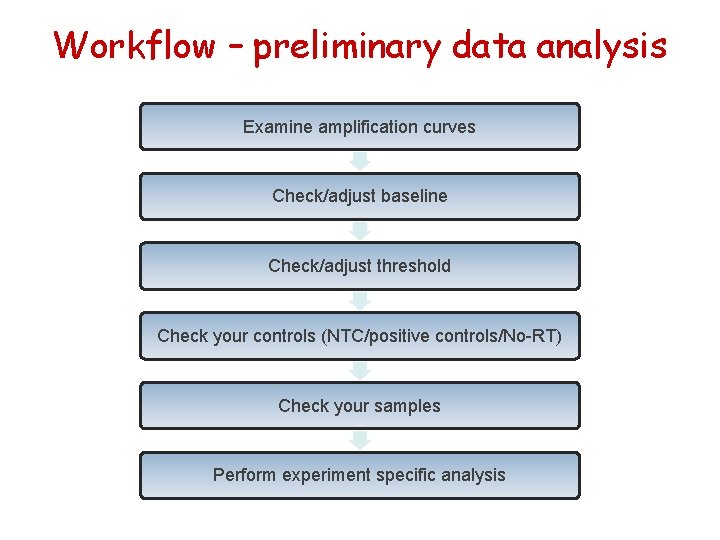
Workflow – preliminary data analysis Examine amplification curves Check/adjust baseline Check/adjust threshold Check your controls (NTC/positive controls/No-RT) Check your samples Perform experiment specific analysis

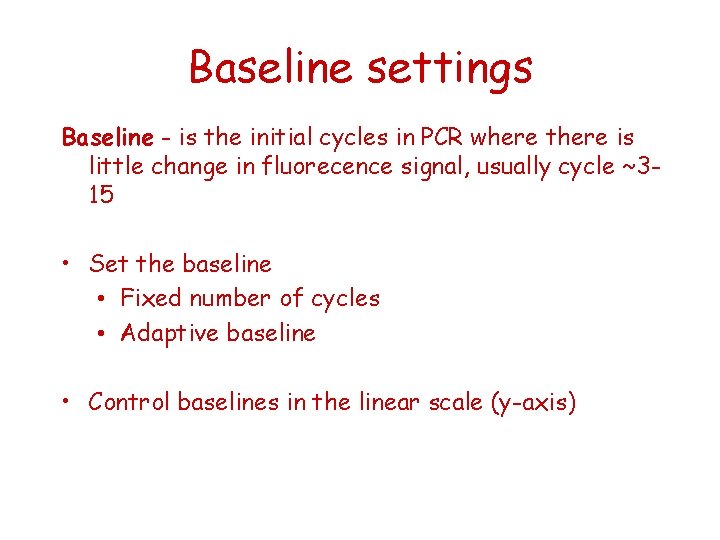
Baseline settings Baseline - is the initial cycles in PCR where there is little change in fluorecence signal, usually cycle ~315 • Set the baseline • Fixed number of cycles • Adaptive baseline • Control baselines in the linear scale (y-axis)

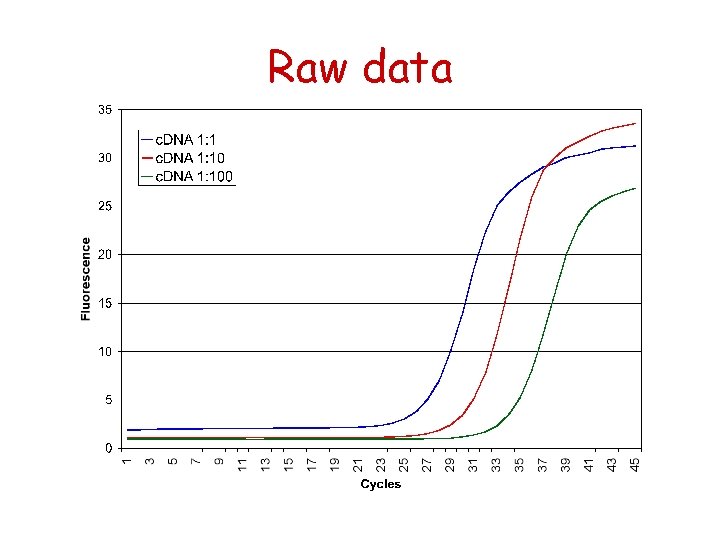
Raw data

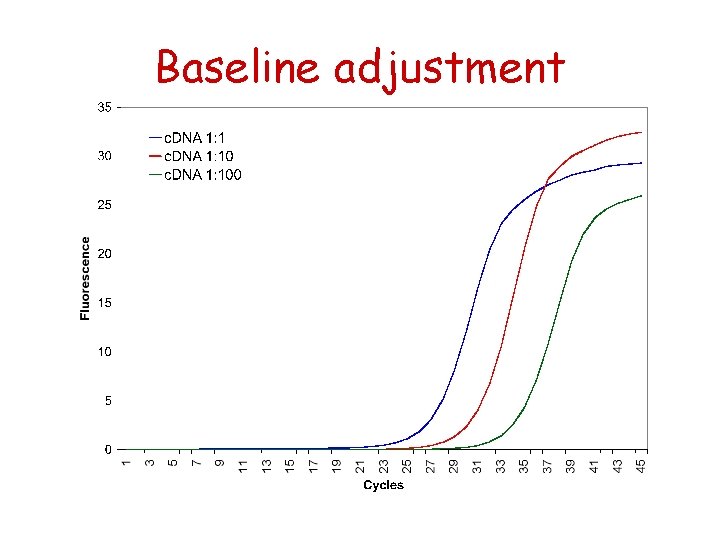
Baseline adjustment

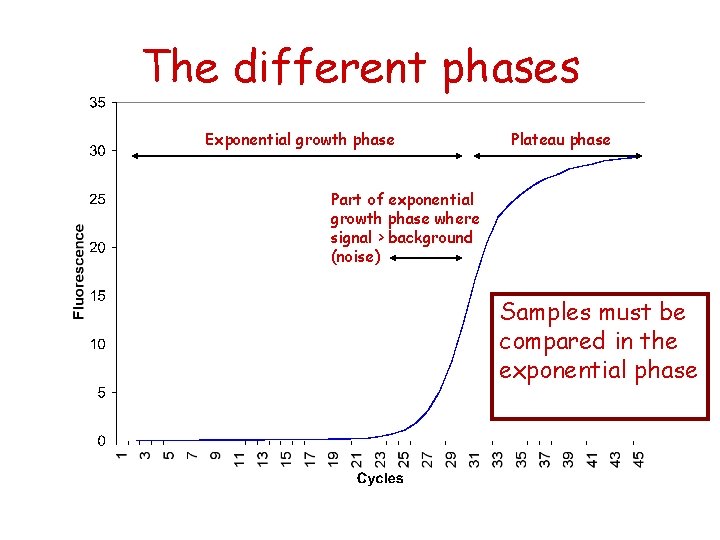
The different phases Exponential growth phase Plateau phase Part of exponential growth phase where signal > background (noise) Samples must be compared in the exponential phase

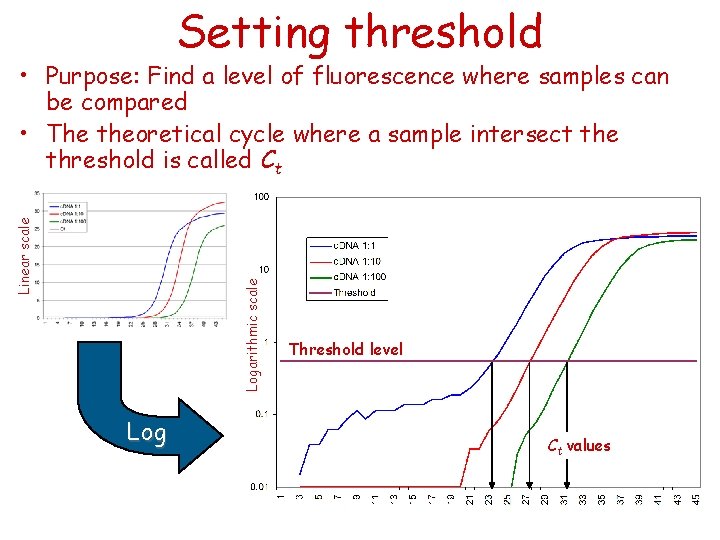
Setting threshold Logarithmic scale Linear scale • Purpose: Find a level of fluorescence where samples can be compared • The theoretical cycle where a sample intersect the threshold is called Ct Log Threshold level Ct values

Setting threshold Ct (threshold cycle): Threshold cycle reflects the cycle number at which the fluorescence generated within a reaction crosses the threshold. It is inversely correlated to the logarithm of the initial copy number. A two-fold difference in copy number should have one Ct difference no matter where threshold is set within the exponential phase

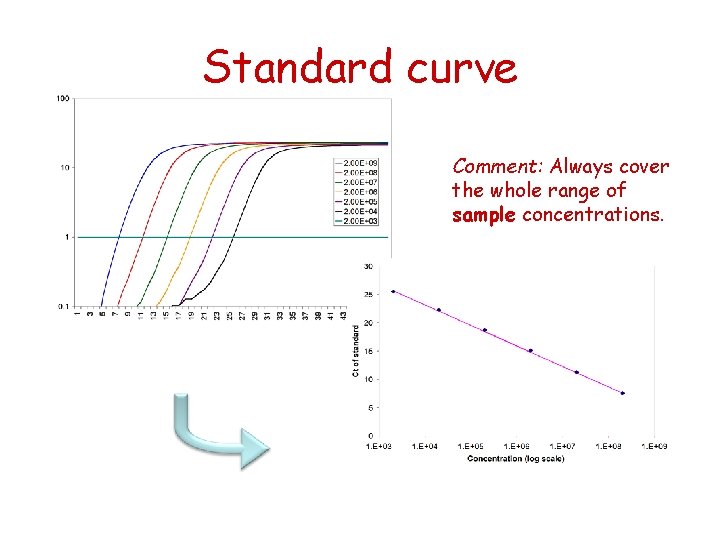
Dilution series and standard curves • Used to control the quality of your assays • Absolute quantification – Standards = Diluted templates of known concentration – Standard curve = Ct of each standard sample is plotted against the known concentration – Used to determine concentrations of unknown samples – Absolute quantification is dependent on the quality of the standard curve

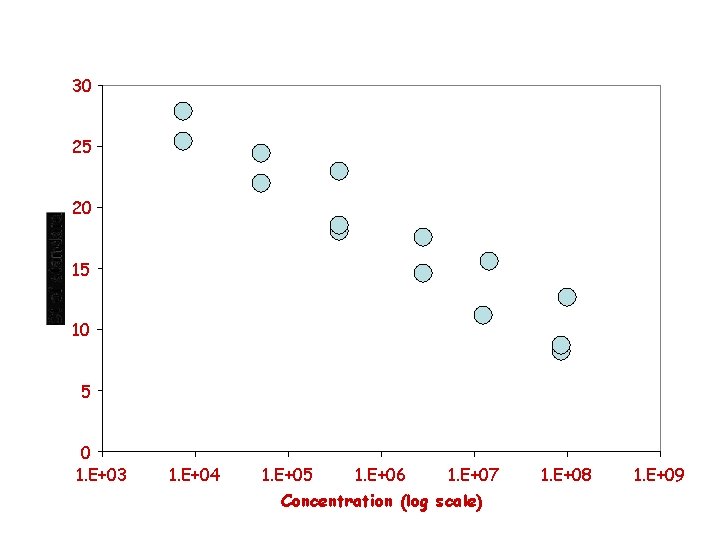
Standard curve Comment: Always cover the whole range of sample concentrations.

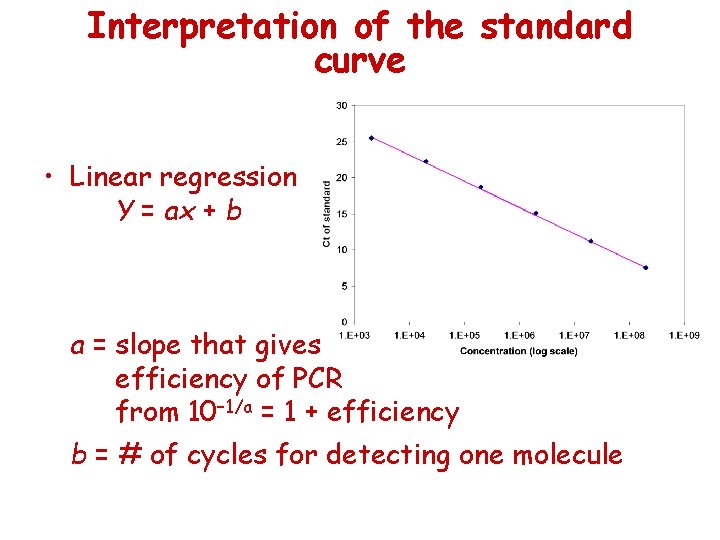
Interpretation of the standard curve • Linear regression Y = ax + b a = slope that gives efficiency of PCR from 10– 1/a = 1 + efficiency b = # of cycles for detecting one molecule

30 25 20 15 10 5 0 1. E+03 1. E+04 1. E+05 1. E+06 1. E+07 Concentration (log scale) 1. E+08 1. E+09

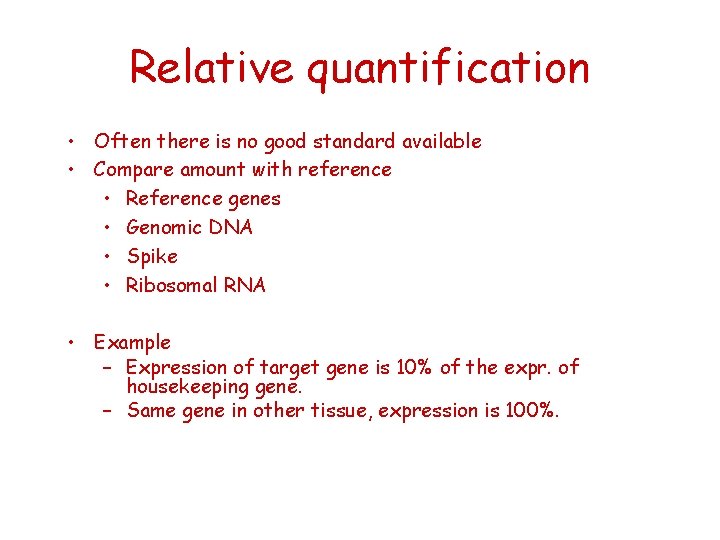
Relative quantification • Often there is no good standard available • Compare amount with reference • Reference genes • Genomic DNA • Spike • Ribosomal RNA • Example – Expression of target gene is 10% of the expr. of housekeeping gene. – Same gene in other tissue, expression is 100%.

Comparing treatments Finding the change in expression of the target gene in the sample compared to the control (no treatment) as a ratio, using a reference gene

Formulas to determine relative expression

MIQUE Nomenclature • MIQUE - Minimum Information for Publication of Quantitative Real-Time PCR experiments Suggested nomenclature • • Reference genes not housekeeping genes Quantification not quantitation Hydrolysis probes not Taq. Man probes Quantification cycle Cq replaces Ct, Cp, TOP

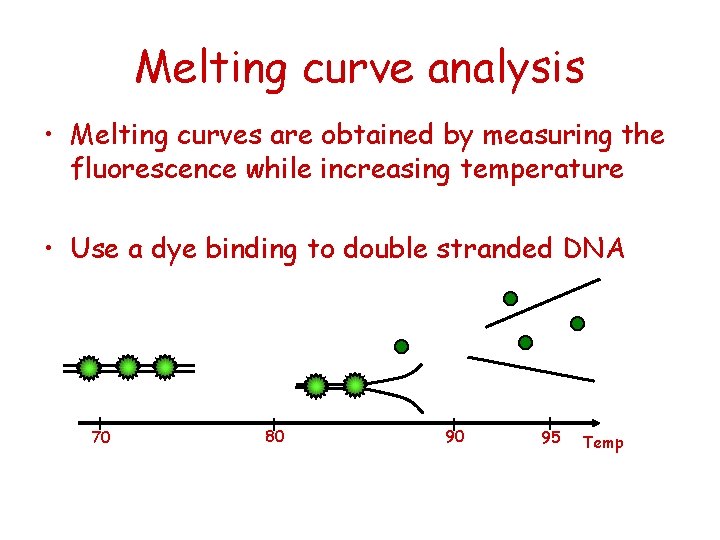
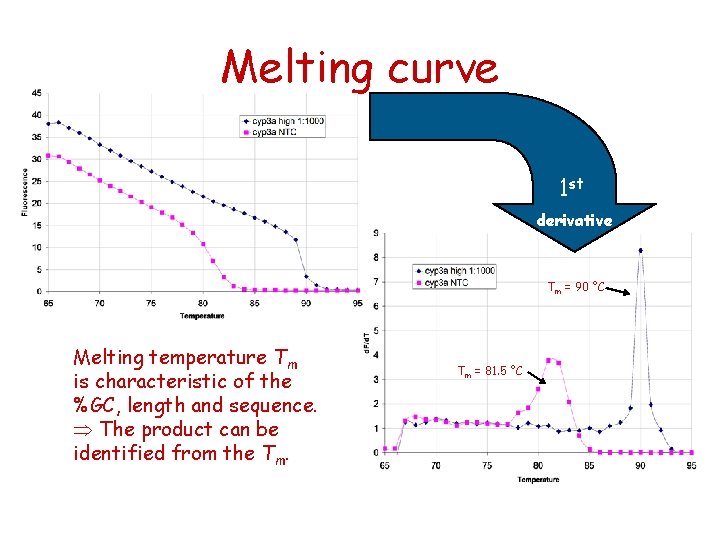
Melting curve analysis • Melting curves are obtained by measuring the fluorescence while increasing temperature • Use a dye binding to double stranded DNA 70 80 90 95 Temp

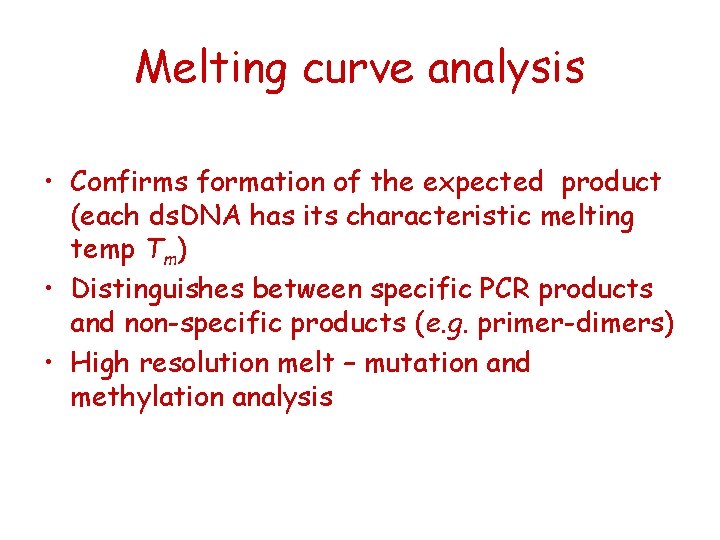
Melting curve analysis • Confirms formation of the expected product (each ds. DNA has its characteristic melting temp Tm) • Distinguishes between specific PCR products and non-specific products (e. g. primer-dimers) • High resolution melt – mutation and methylation analysis

Melting curve 1 st derivative Tm = 90 °C Melting temperature Tm is characteristic of the %GC, length and sequence. The product can be identified from the Tm. Tm = 81. 5 °C

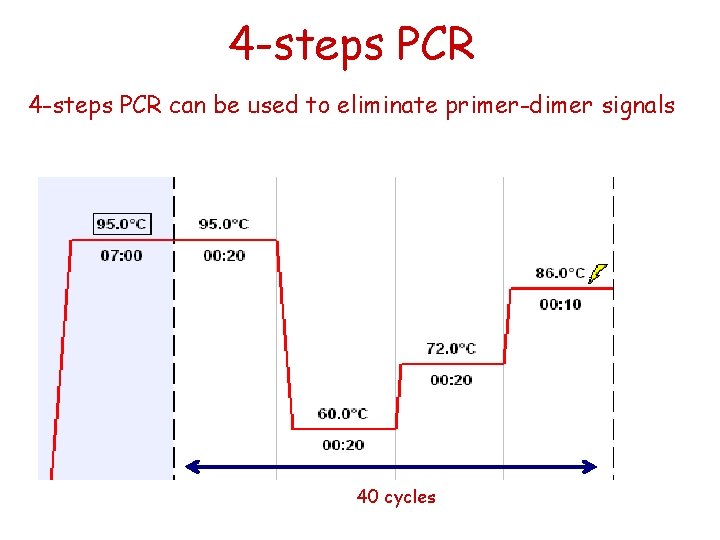
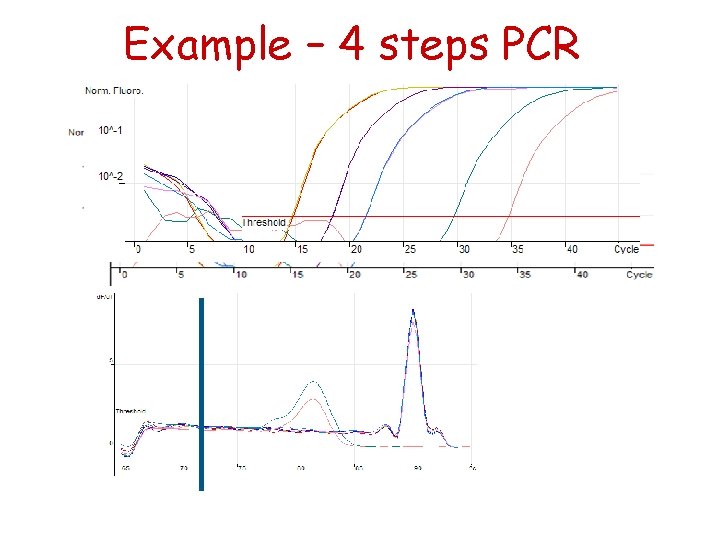
4 -steps PCR can be used to eliminate primer-dimer signals 40 cycles

Example – 4 steps PCR



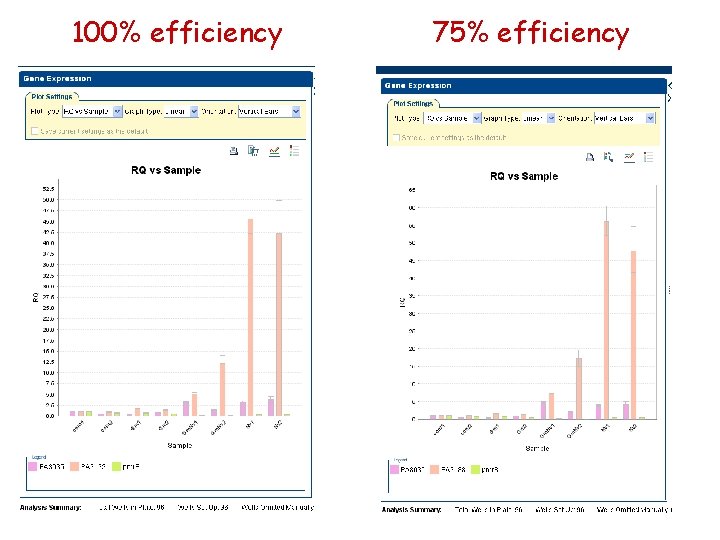
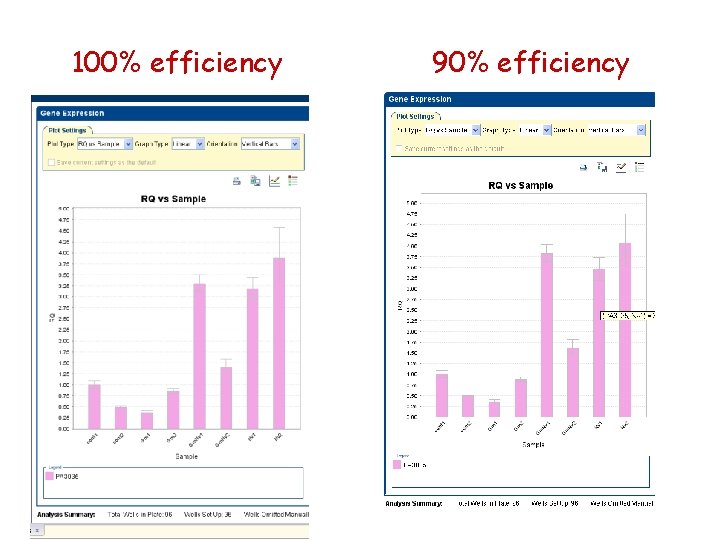
100% efficiency 75% efficiency

100% efficiency 90% efficiency

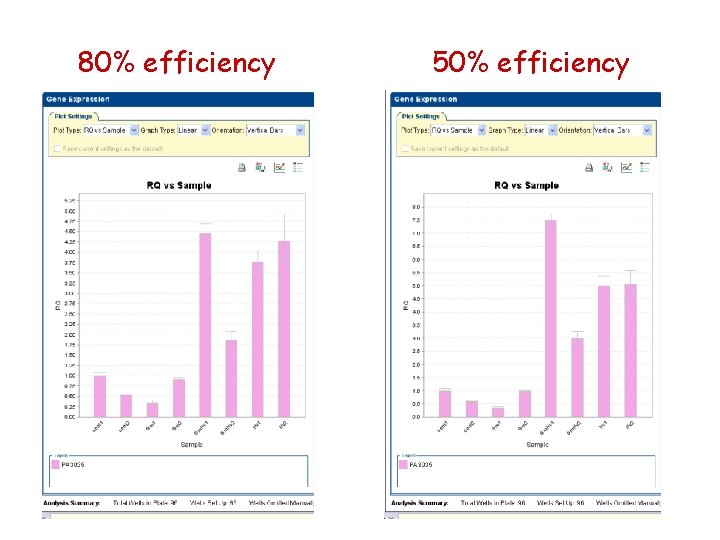
80% efficiency 50% efficiency

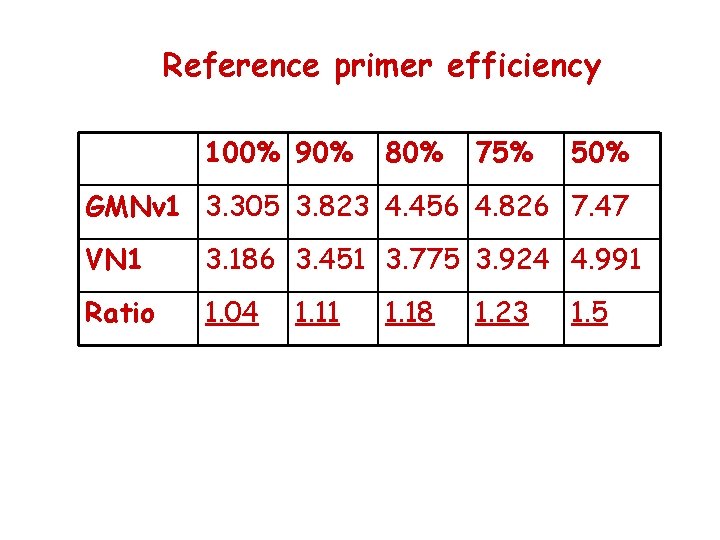
Reference primer efficiency 100% 90% 80% 75% 50% GMNv 1 3. 305 3. 823 4. 456 4. 826 7. 47 VN 1 3. 186 3. 451 3. 775 3. 924 4. 991 Ratio 1. 04 1. 11 1. 18 1. 23 1. 5

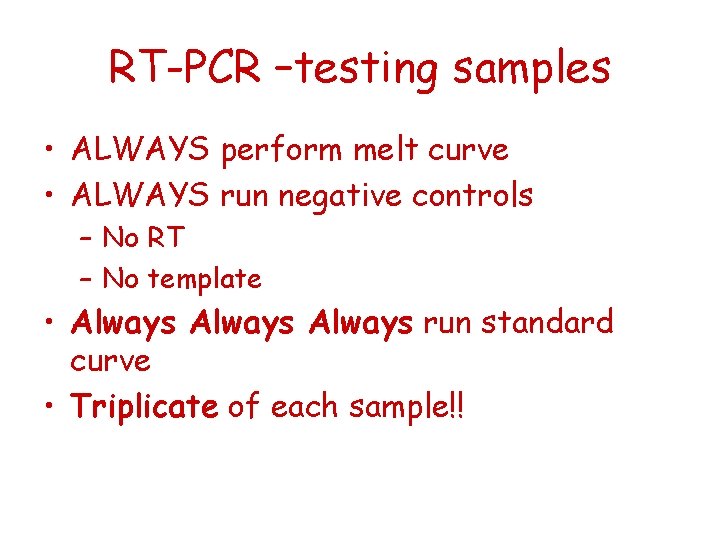
RT-PCR –testing samples • ALWAYS perform melt curve • ALWAYS run negative controls – No RT – No template • Always run standard curve • Triplicate of each sample!!

Requirements for RT-PCR Experiment • • • Always perform standard curve All samples in triplicate NTC control No RT control Prepare mix without c. DNA; add this to each tube separately Divide plate by gene and not sample Do not need reference gene on every plate Melt Curve Check RNA- otherwise don’t bother with experiment Do not rely on only 1 reference gene- check more than one per project • Every project is different! • Don’t be afraid to ask me questions, especially BEFORE starting the project.

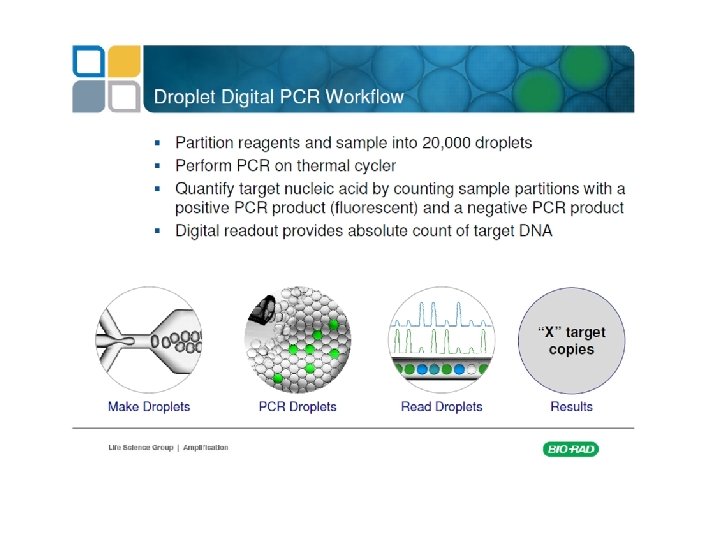
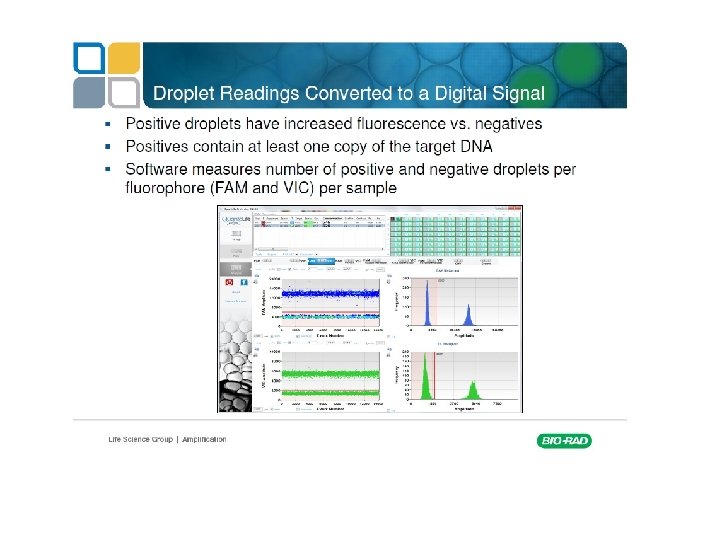
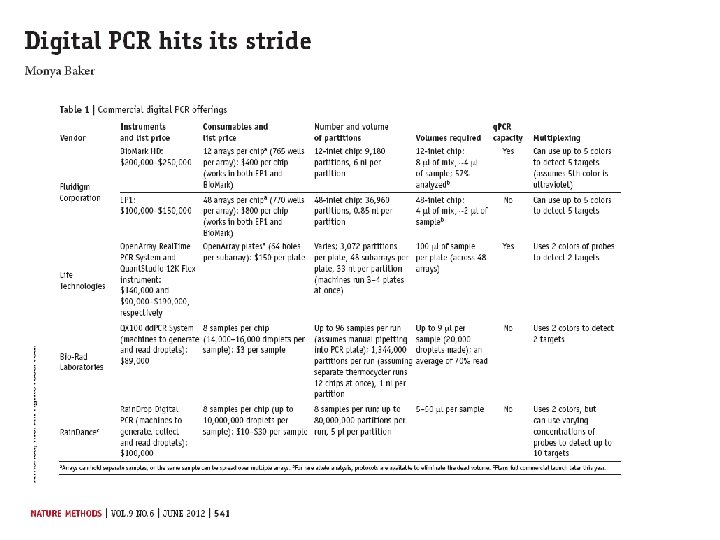
Digital PCR From Relative quantity to absolute quantity

http: //cgs. hku. hk/portal/files/GRC/Events/S eminars/20120502/qx 100%20 digital %20 pcr%20 introduction%20 mikki%20 koo. pdf

Commercially available machines Fluidigm Quanta. Soft (Life Technologies)

Rain Dance Bio Rad QX 100








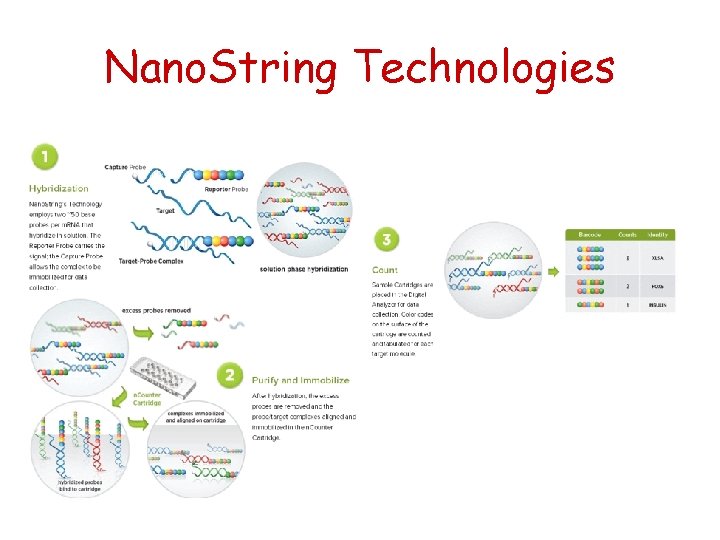
Nano. String Technologies Direct multiplexed measurement of gene expression with color-coded probe pairs NATURE BIOTECHNOLOGY VOLUME 26 NUMBER 3 MARCH 2008

Nano. String Technologies
 An introduction to shakespeare and romeo and juliet part 1
An introduction to shakespeare and romeo and juliet part 1 Part whole model subtraction
Part whole model subtraction Unit ratio definition
Unit ratio definition Brainpop ratios
Brainpop ratios Technical description
Technical description What are the parts of a bar
What are the parts of a bar The part of a shadow surrounding the darkest part
The part of a shadow surrounding the darkest part Part to part variation
Part to part variation Epic poem the odyssey
Epic poem the odyssey An introduction to the odyssey by david adams leeming
An introduction to the odyssey by david adams leeming What rules must an epic follow?
What rules must an epic follow? The odyssey and epic poetry: an introduction, part 1
The odyssey and epic poetry: an introduction, part 1 The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1 Homers epic poem
Homers epic poem Introducing the odyssey
Introducing the odyssey The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1 The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1 The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1 Illustration of the steps in restriction digestion and pcr
Illustration of the steps in restriction digestion and pcr Illustrate the steps in restriction digestion and pcr
Illustrate the steps in restriction digestion and pcr Hamlet part 1 an introduction to elizabethan theater
Hamlet part 1 an introduction to elizabethan theater First part of introduction
First part of introduction Hamlet, part 1: an introduction to elizabethan theater
Hamlet, part 1: an introduction to elizabethan theater Rcog training portfolio
Rcog training portfolio Yeast colony pcr
Yeast colony pcr Pcr
Pcr Retrotranscripción
Retrotranscripción Rcp extracorpórea
Rcp extracorpórea Purpose of pcr
Purpose of pcr Polymerase chain reaction uses
Polymerase chain reaction uses Biorad pcr master mix
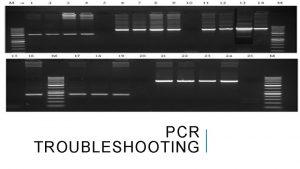
Biorad pcr master mix Pcr troubleshooting multiple bands
Pcr troubleshooting multiple bands Objectives of pcr
Objectives of pcr Aplication of pcr
Aplication of pcr Pcr
Pcr Qpcr 2-ddct
Qpcr 2-ddct Pcr abi
Pcr abi Drug resistance training
Drug resistance training Pcr nobel prize
Pcr nobel prize Ecr engineering change request
Ecr engineering change request Crystal digital pcr
Crystal digital pcr Missy baker
Missy baker Pcr annealing temperature too high
Pcr annealing temperature too high Pcr biology definition
Pcr biology definition Site:slidetodoc.com
Site:slidetodoc.com Pcr basics worksheet answers minipcr
Pcr basics worksheet answers minipcr Pcr
Pcr Pcr gif animation
Pcr gif animation Reactantes de fase aguda
Reactantes de fase aguda Pcr file fullprof
Pcr file fullprof Slan real time pcr
Slan real time pcr Basics of microbiology
Basics of microbiology Xét nghiệm pcr
Xét nghiệm pcr Rflp animation
Rflp animation