Introduction to Organic Chemistry Session 28 Ciencias de






![Bibliography • Catholic University of Cordoba. (2003). Faculty of Chemistry Sciences [Image]. Catholic University Bibliography • Catholic University of Cordoba. (2003). Faculty of Chemistry Sciences [Image]. Catholic University](https://slidetodoc.com/presentation_image/11800d1a2a540e12a5753766fd976947/image-10.jpg)
- Slides: 10

Introduction to Organic Chemistry (Session 28) Ciencias de la tierra Universidad Católica de Córdoba (2003)

Organic Chemistry • Branch of chemistry that studies the carbon compounds. • The number of natural and synthetic organic compounds known to date is over ten million. Ciencias de la tierra Universidad Católica de Córdoba (2003)

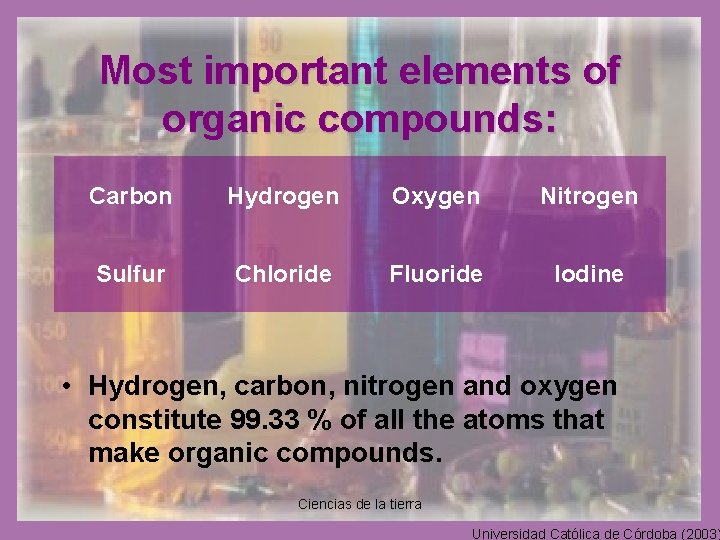
Most important elements of organic compounds: Carbon Hydrogen Oxygen Nitrogen Sulfur Chloride Fluoride Iodine • Hydrogen, carbon, nitrogen and oxygen constitute 99. 33 % of all the atoms that make organic compounds. Ciencias de la tierra Universidad Católica de Córdoba (2003)

Other elements in the organic compounds: Phosphorus Magnesium Cobalt Calcium Manganese Molybdenum Iron Boron Ciencias de la tierra Universidad Católica de Córdoba (2003)

Valence Electrons • They are the electrons that are found in the last cap of the atom. • By their electronic configuration we can know the number of valence electrons that an atom has and with this the number of bonds that they can form. For example: – Hydrogen Atomic Number = 1 – Electronic configuration 1 s 1 ____ • They can only form one covalent bonds because they present one orbital s half full. Ciencias de la tierra Universidad Católica de Córdoba (2003)

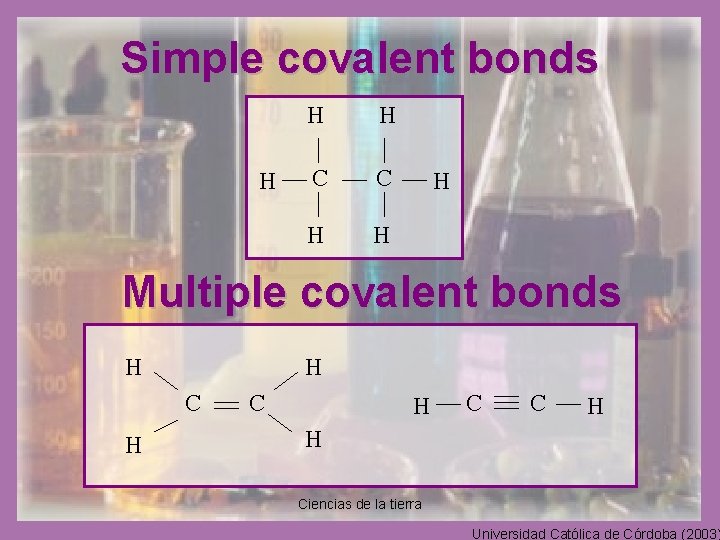
Bonds in the organic compounds • The covalent bonds is the only predominant chemical bonds of the organic compounds. • The covalent bonds is formed when the atoms are bonded share their electrons. • In the organic compounds we can find two types of covalent bonds: – Simple covalent, when two electrons are shared within atoms. – Multiple covalent, when more then two electrons (four or six electrons) within atoms. Ciencias de la tierra Universidad Católica de Córdoba (2003)

Simple covalent bonds H H H C C H H H Multiple covalent bonds H H C H C C H H Ciencias de la tierra Universidad Católica de Córdoba (2003)

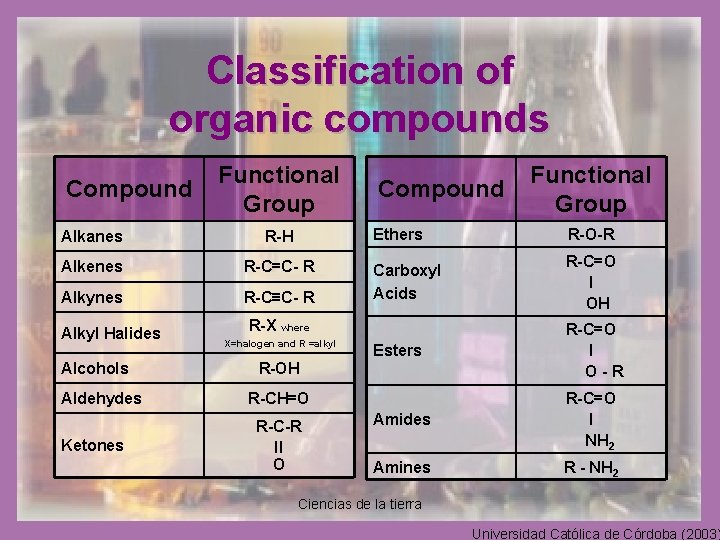
Classification of organic compounds • Organic compounds have been classified according to the group of atoms that characterize them and of which their chemical and chemical properties depend greatly on. • This group of atoms has been named functional group. Ciencias de la tierra Universidad Católica de Córdoba (2003)

Classification of organic compounds Compound Functional Group Alkanes R-H Alkenes R-C=C- R Alkynes R-C≡C- R Alkyl Halides Alcohols Aldehydes Ketones Compound Ethers R-O-R Carboxyl Acids R-C=O ׀ OH Esters R-C=O ׀ O-R Amides R-C=O ׀ NH 2 Amines R - NH 2 R-X where X=halogen and R =alkyl R-OH R-CH=O R-C-R ׀׀ O Functional Group Ciencias de la tierra Universidad Católica de Córdoba (2003)
![Bibliography Catholic University of Cordoba 2003 Faculty of Chemistry Sciences Image Catholic University Bibliography • Catholic University of Cordoba. (2003). Faculty of Chemistry Sciences [Image]. Catholic University](https://slidetodoc.com/presentation_image/11800d1a2a540e12a5753766fd976947/image-10.jpg)
Bibliography • Catholic University of Cordoba. (2003). Faculty of Chemistry Sciences [Image]. Catholic University of Cordoba. Recuperated on May 27 2003 from the World Wide Web: http: //www. uccor. edu. ar/facultades. php? fa cultad=5 • Rakoff, H. et al. (1992). Fundamental Organic Chemistry. 1 st ed. Mexico: LIMUSA Editorial Ciencias de la tierra Universidad Católica de Córdoba (2003)