Introduction to Organic Chemistry Alkanes Functional Groups Elements






- Slides: 49

Introduction to Organic Chemistry: Alkanes Functional Groups

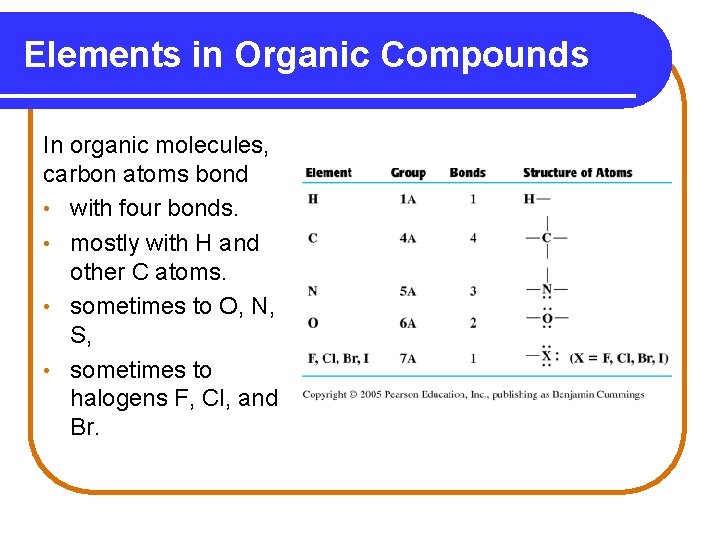
Elements in Organic Compounds In organic molecules, carbon atoms bond • with four bonds. • mostly with H and other C atoms. • sometimes to O, N, S, • sometimes to halogens F, Cl, and Br.

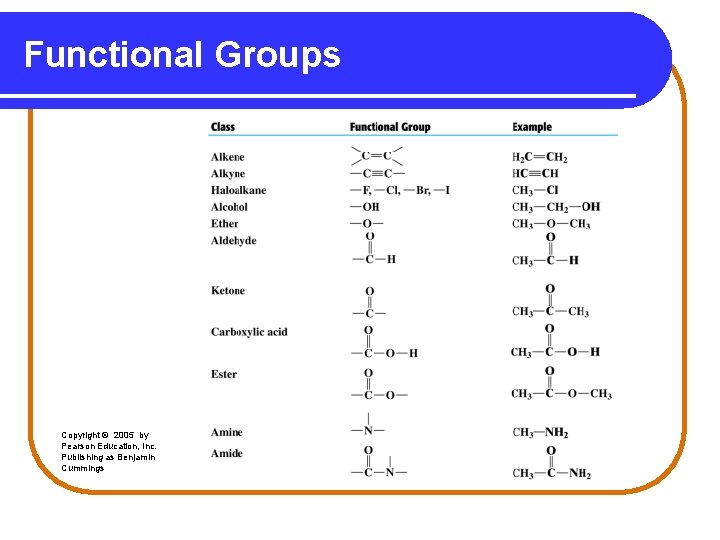
Functional Groups Functional groups are • a characteristic feature of organic molecules that behave in a predictable way. • composed of an atom or group of atoms. • groups that replace a hydrogen atom in the corresponding alkane. • a way to classify families of organic compounds.

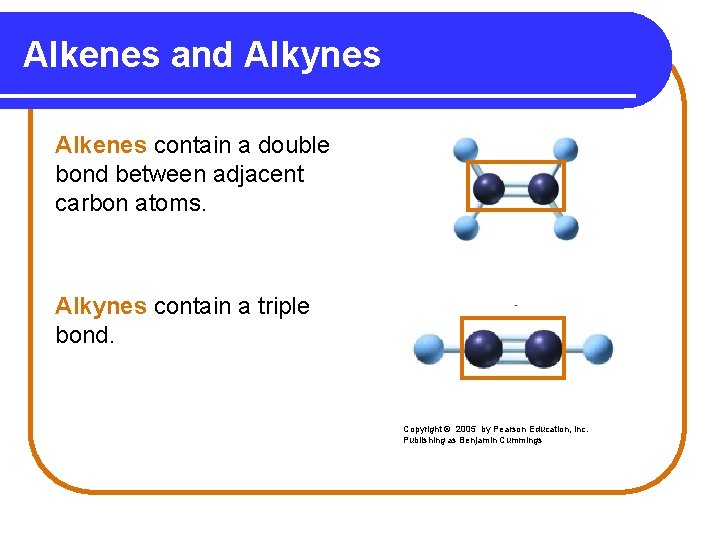
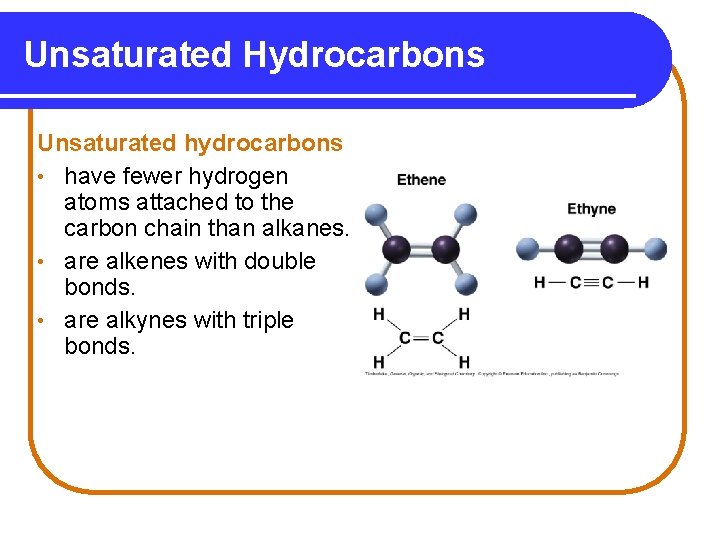
Alkenes and Alkynes Alkenes contain a double bond between adjacent carbon atoms. Alkynes contain a triple bond. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings

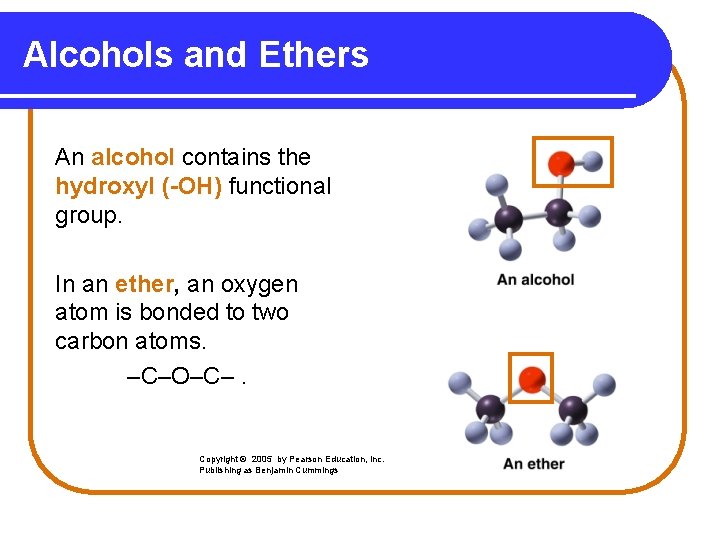
Alcohols and Ethers An alcohol contains the hydroxyl (-OH) functional group. In an ether, an oxygen atom is bonded to two carbon atoms. –C–O–C–. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings

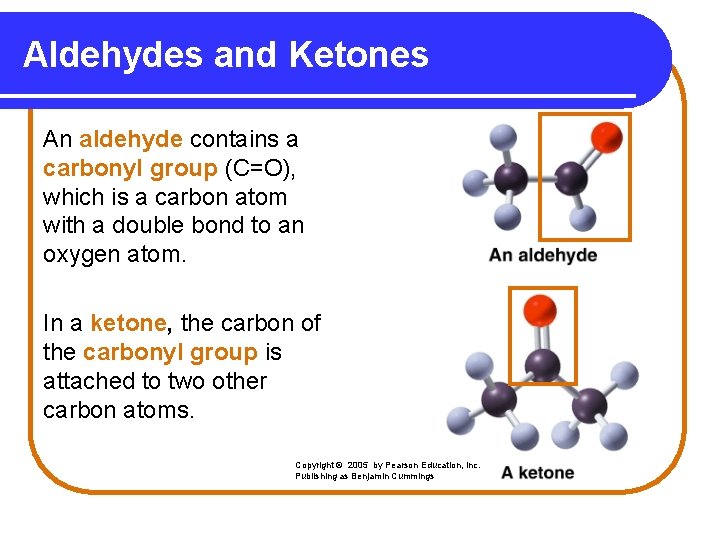
Aldehydes and Ketones An aldehyde contains a carbonyl group (C=O), which is a carbon atom with a double bond to an oxygen atom. In a ketone, the carbon of the carbonyl group is attached to two other carbon atoms. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings

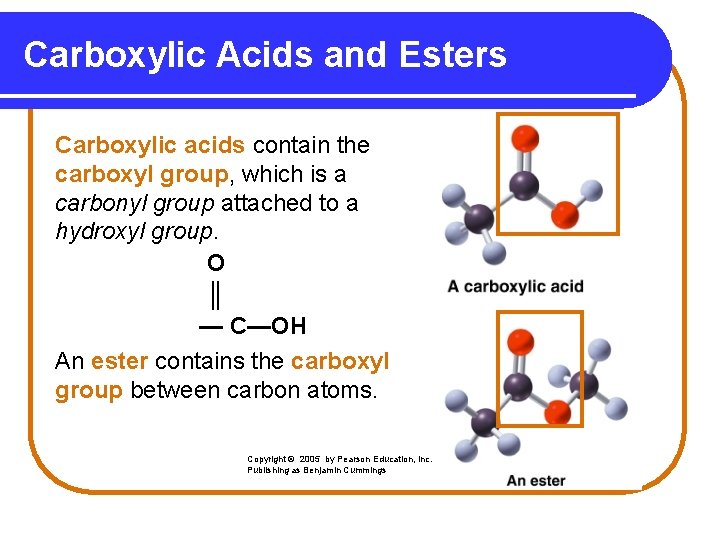
Carboxylic Acids and Esters Carboxylic acids contain the carboxyl group, which is a carbonyl group attached to a hydroxyl group. O ║ — C—OH An ester contains the carboxyl group between carbon atoms. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings

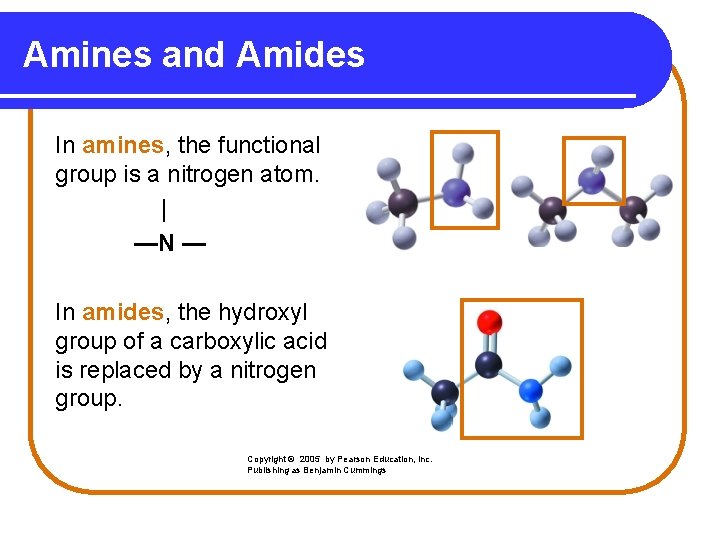
Amines and Amides In amines, the functional group is a nitrogen atom. | —N — In amides, the hydroxyl group of a carboxylic acid is replaced by a nitrogen group. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings

Functional Groups Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings

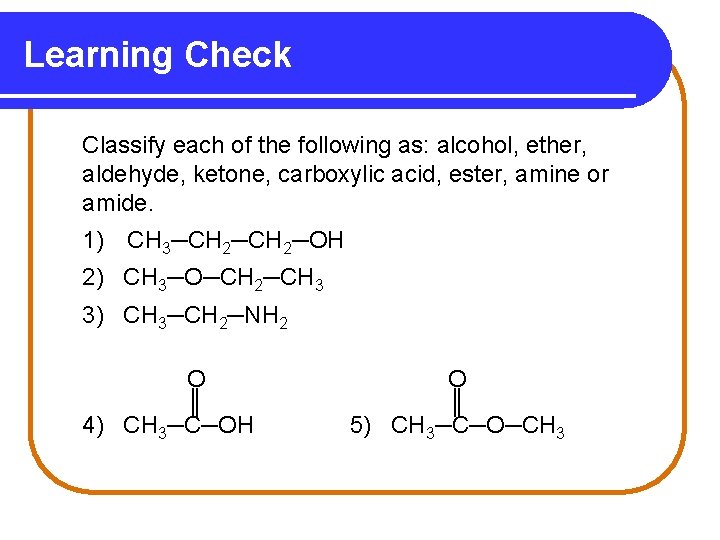
Learning Check Classify each of the following as: alcohol, ether, aldehyde, ketone, carboxylic acid, ester, amine or amide. 1) CH 3─CH 2─OH 2) CH 3─O─CH 2─CH 3 3) CH 3─CH 2─NH 2 O ║ 4) CH 3─C─OH O ║ 5) CH 3─C─O─CH 3

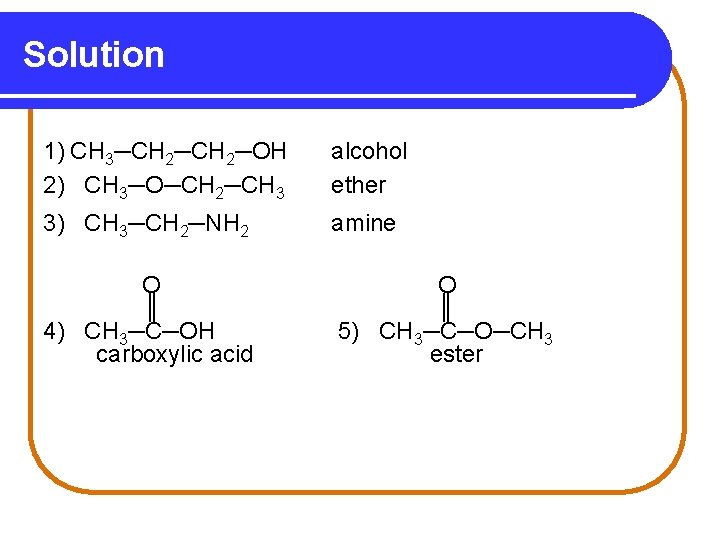
Solution 1) CH 3─CH 2─OH 2) CH 3─O─CH 2─CH 3 alcohol ether 3) CH 3─CH 2─NH 2 amine O ║ 4) CH 3─C─OH carboxylic acid O ║ 5) CH 3─C─O─CH 3 ester

Some functional groups in consumer products BENGAY l Camphor – a ketone l Menthol – an alcohol l Methyl salicylate – an ester and a phenol l Neopsporin l Miconazole – many functional groups (ether, chloro) l Dimethyl ether propellent l Halls cough l Menthol- an alcohol l

Some functional groups in spices Nutmeg l Myristicin – a polyether, an aklene, benzene ring l Elemicin l Safrole l Anise l Anethole – an ether, an alkene, benzene ring l Caraway seeds l d-Carvone – a ketone l Spearmint l l-Carvone – a ketone l

Unsaturated Hydrocarbons Alkenes and Alkynes

Saturated Hydrocarbons Saturated hydrocarbons • have the maximum number of hydrogen atoms attached to each carbon atom. • are alkanes and cycloalkanes with single C-C bonds. CH 3—CH 2—CH 3

Unsaturated Hydrocarbons Unsaturated hydrocarbons • have fewer hydrogen atoms attached to the carbon chain than alkanes. • are alkenes with double bonds. • are alkynes with triple bonds.

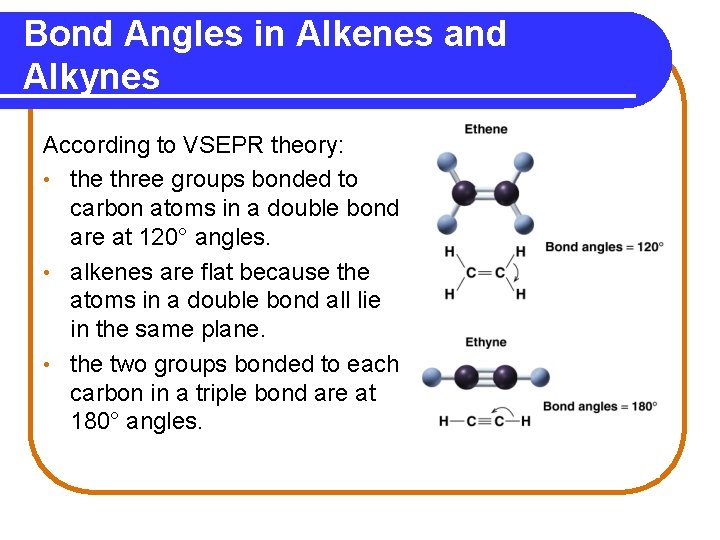
Bond Angles in Alkenes and Alkynes According to VSEPR theory: • the three groups bonded to carbon atoms in a double bond are at 120° angles. • alkenes are flat because the atoms in a double bond all lie in the same plane. • the two groups bonded to each carbon in a triple bond are at 180° angles.

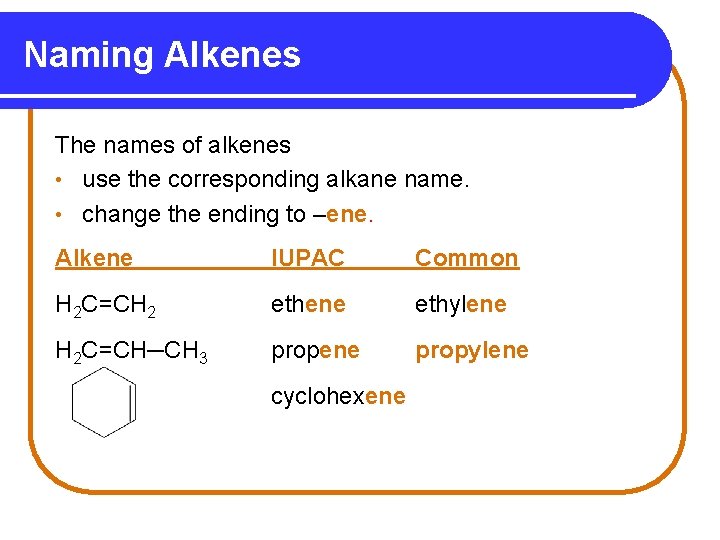
Naming Alkenes The names of alkenes • use the corresponding alkane name. • change the ending to –ene. Alkene IUPAC Common H 2 C=CH 2 ethene ethylene H 2 C=CH─CH 3 propene propylene cyclohexene

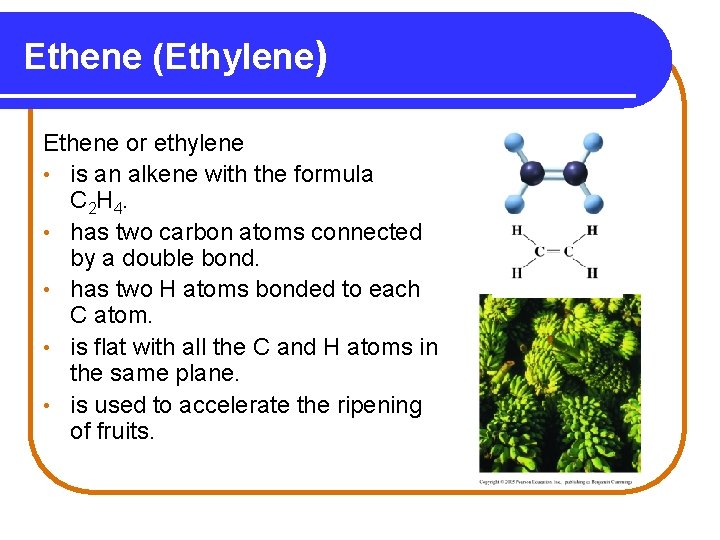
Ethene (Ethylene) Ethene or ethylene • is an alkene with the formula C 2 H 4. • has two carbon atoms connected by a double bond. • has two H atoms bonded to each C atom. • is flat with all the C and H atoms in the same plane. • is used to accelerate the ripening of fruits.

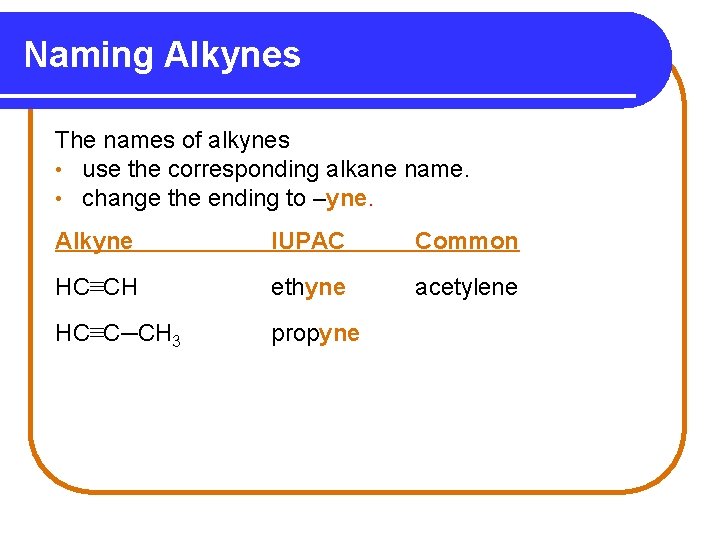
Naming Alkynes The names of alkynes • use the corresponding alkane name. • change the ending to –yne. Alkyne IUPAC Common HC≡CH ethyne acetylene HC≡C─CH 3 propyne

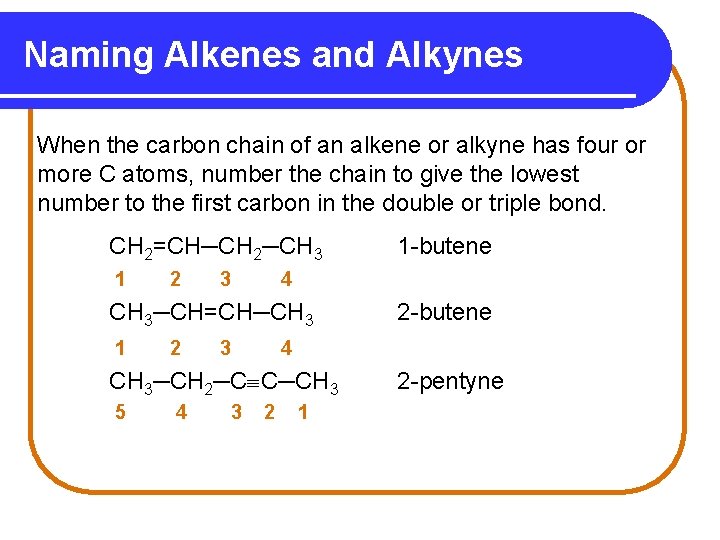
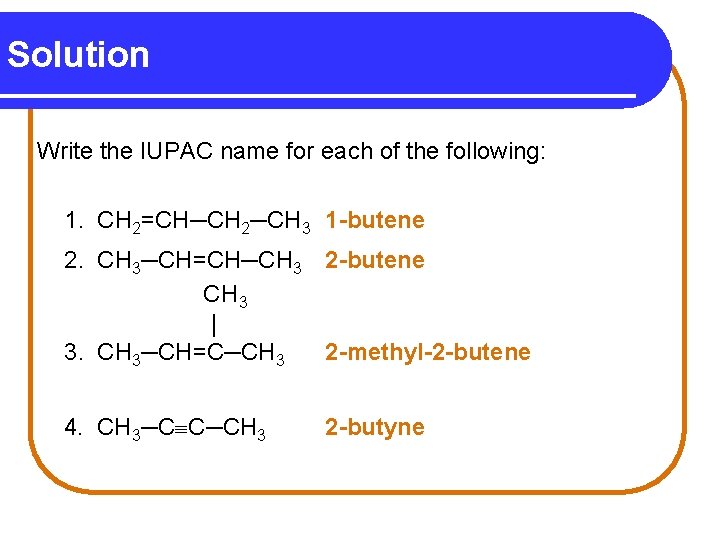
Naming Alkenes and Alkynes When the carbon chain of an alkene or alkyne has four or more C atoms, number the chain to give the lowest number to the first carbon in the double or triple bond. CH 2=CH─CH 2─CH 3 1 2 3 4 CH 3─CH=CH─CH 3 1 2 3 4 3 2 -butene 4 CH 3─CH 2─C C─CH 3 5 1 -butene 2 1 2 -pentyne

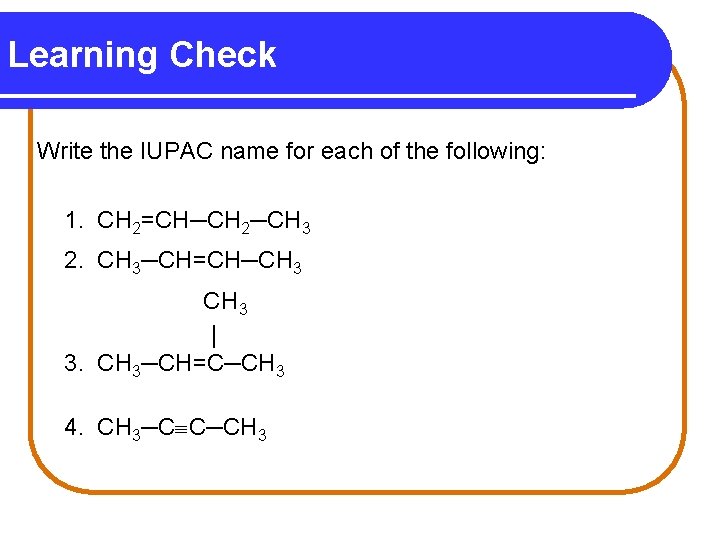
Learning Check Write the IUPAC name for each of the following: 1. CH 2=CH─CH 2─CH 3 2. CH 3─CH=CH─CH 3 | 3. CH 3─CH=C─CH 3 4. CH 3─C C─CH 3

Solution Write the IUPAC name for each of the following: 1. CH 2=CH─CH 2─CH 3 1 -butene 2. CH 3─CH=CH─CH 3 2 -butene CH 3 | 3. CH 3─CH=C─CH 3 2 -methyl-2 -butene 4. CH 3─C C─CH 3 2 -butyne

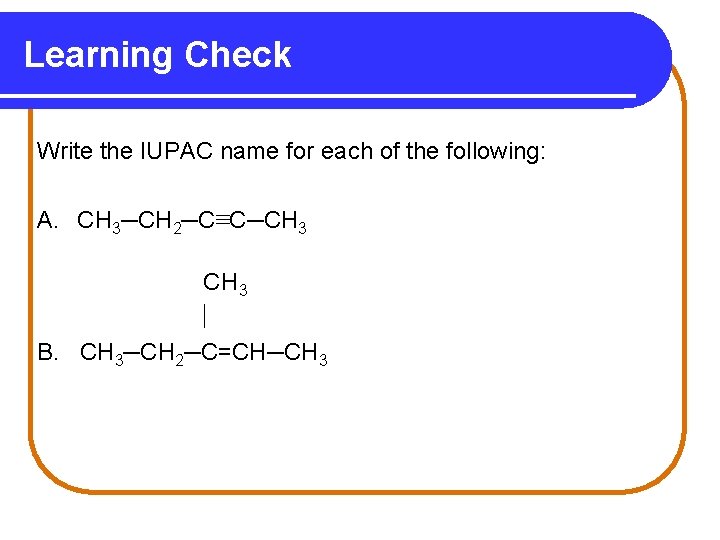
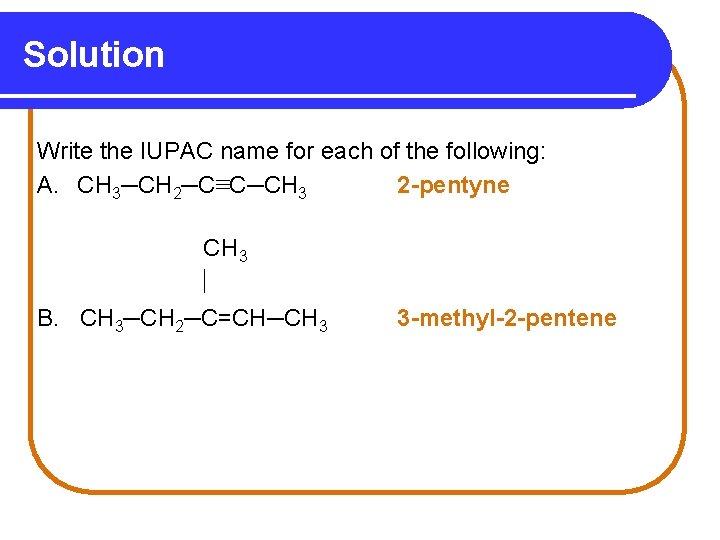
Learning Check Write the IUPAC name for each of the following: A. CH 3─CH 2─C≡C─CH 3 B. CH 3─CH 2─C=CH─CH 3

Solution Write the IUPAC name for each of the following: A. CH 3─CH 2─C≡C─CH 3 2 -pentyne CH 3 B. CH 3─CH 2─C=CH─CH 3 3 -methyl-2 -pentene

Chapter 11 Unsaturated Hydrocarbons 11. 2 Cis-Trans Isomers

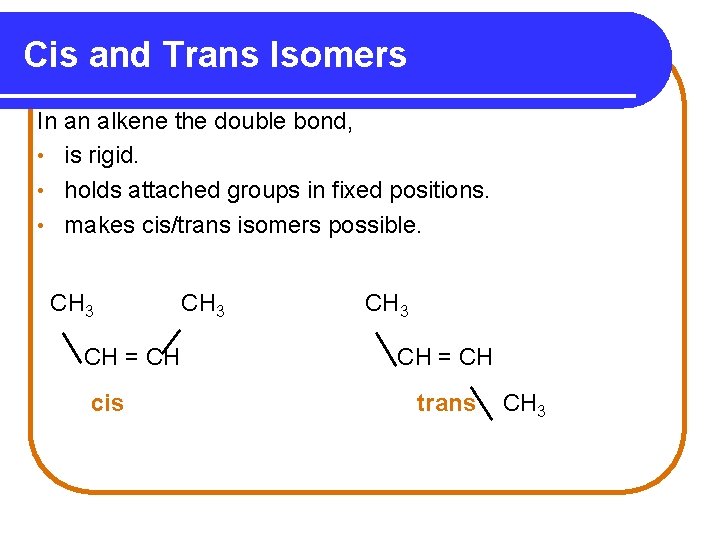
Cis and Trans Isomers In an alkene the double bond, • is rigid. • holds attached groups in fixed positions. • makes cis/trans isomers possible. CH 3 CH = CH cis CH 3 CH = CH trans CH 3

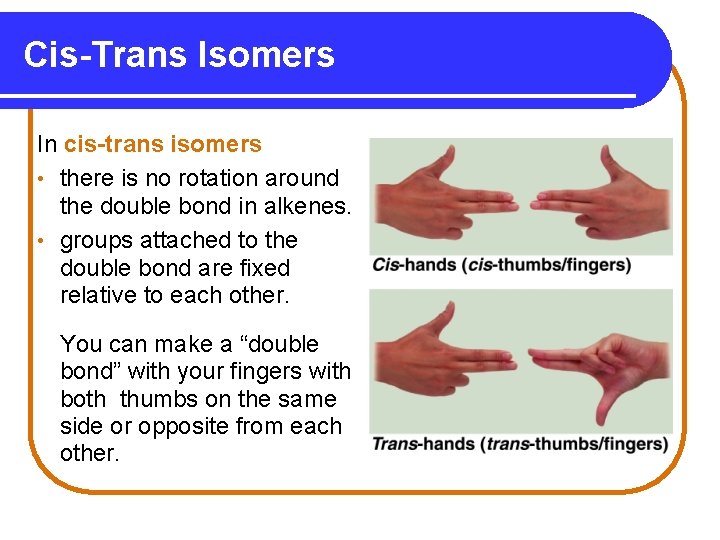
Cis-Trans Isomers In cis-trans isomers • there is no rotation around the double bond in alkenes. • groups attached to the double bond are fixed relative to each other. You can make a “double bond” with your fingers with both thumbs on the same side or opposite from each other.

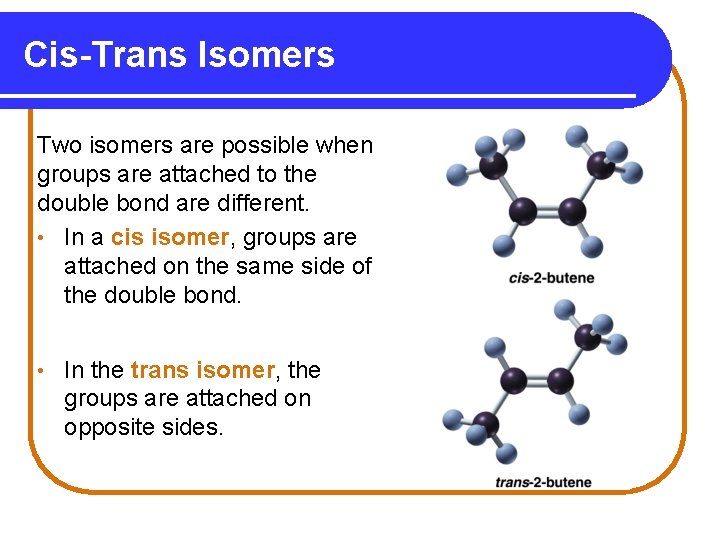
Cis-Trans Isomers Two isomers are possible when groups are attached to the double bond are different. • In a cis isomer, groups are attached on the same side of the double bond. • In the trans isomer, the groups are attached on opposite sides.

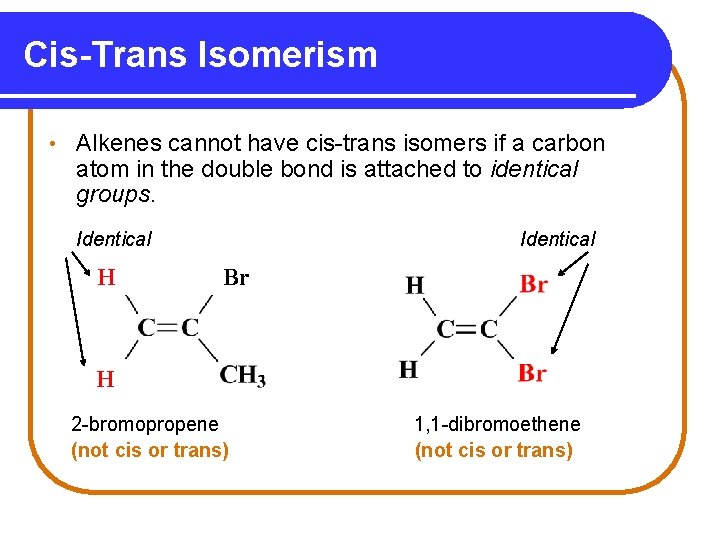
Cis-Trans Isomerism • Alkenes cannot have cis-trans isomers if a carbon atom in the double bond is attached to identical groups. Identical H Identical Br H H 2 -bromopropene (not cis or trans) 1, 1 -dibromoethene (not cis or trans)

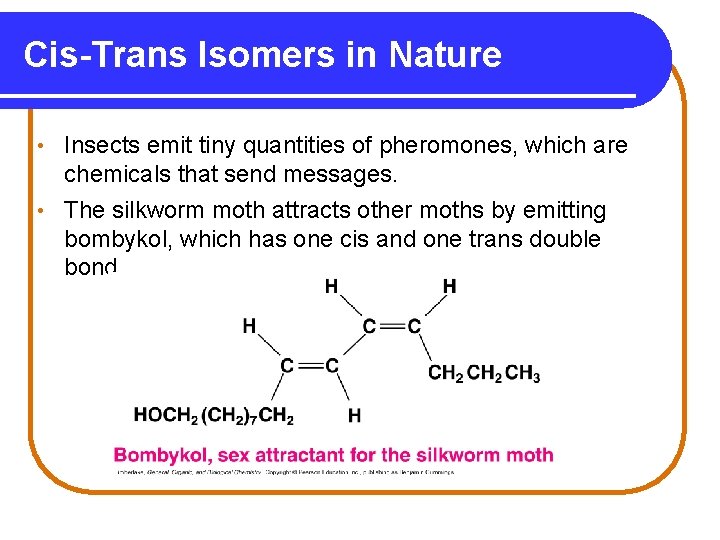
Cis-Trans Isomers in Nature Insects emit tiny quantities of pheromones, which are chemicals that send messages. • The silkworm moth attracts other moths by emitting bombykol, which has one cis and one trans double bond. •

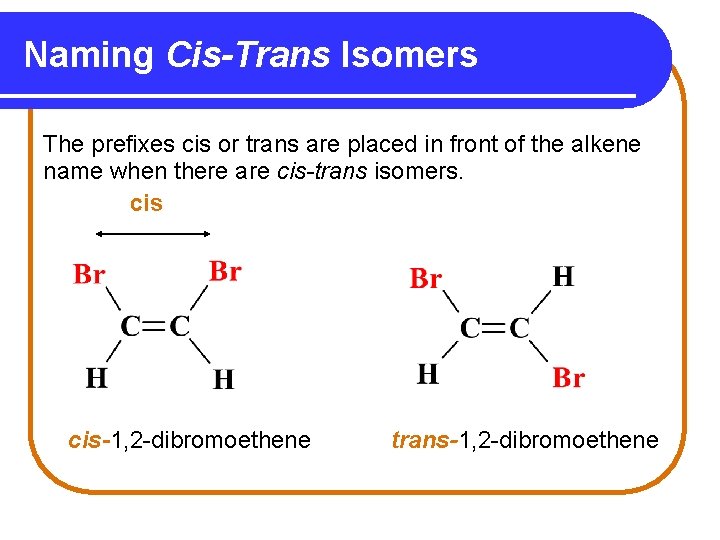
Naming Cis-Trans Isomers The prefixes cis or trans are placed in front of the alkene name when there are cis-trans isomers. cis-1, 2 -dibromoethene trans-1, 2 -dibromoethene

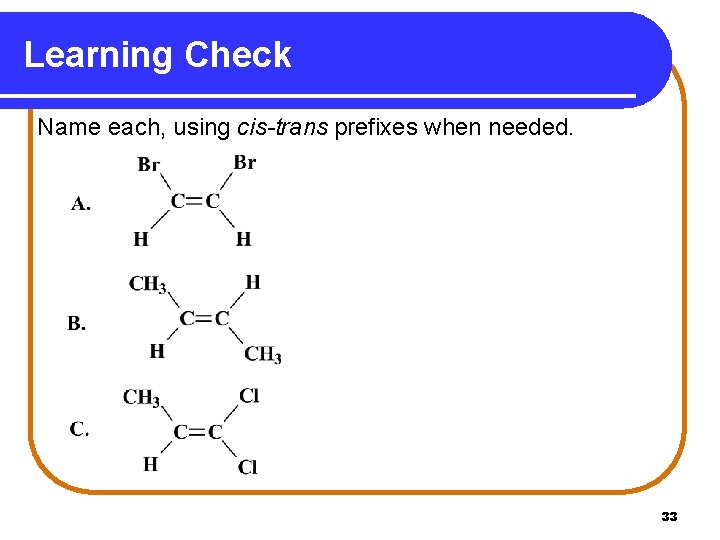
Learning Check Name each, using cis-trans prefixes when needed. 33

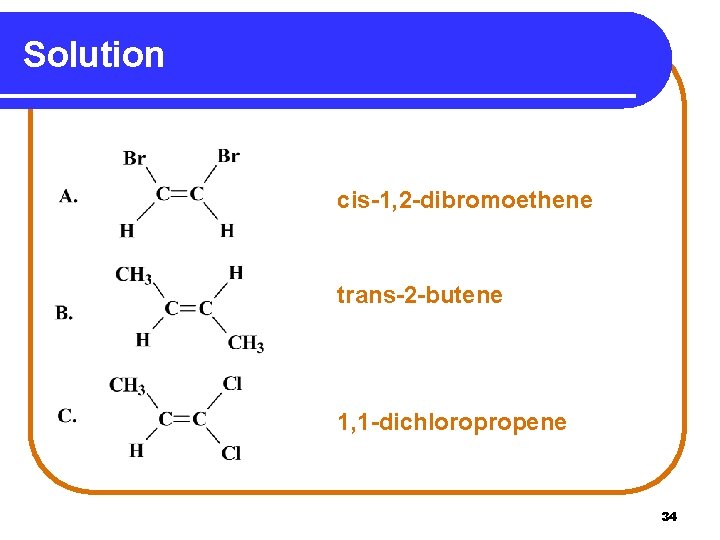
Solution cis-1, 2 -dibromoethene trans-2 -butene 1, 1 -dichloropropene 34

Unsaturated Hydrocarbons Addition Reactions 35

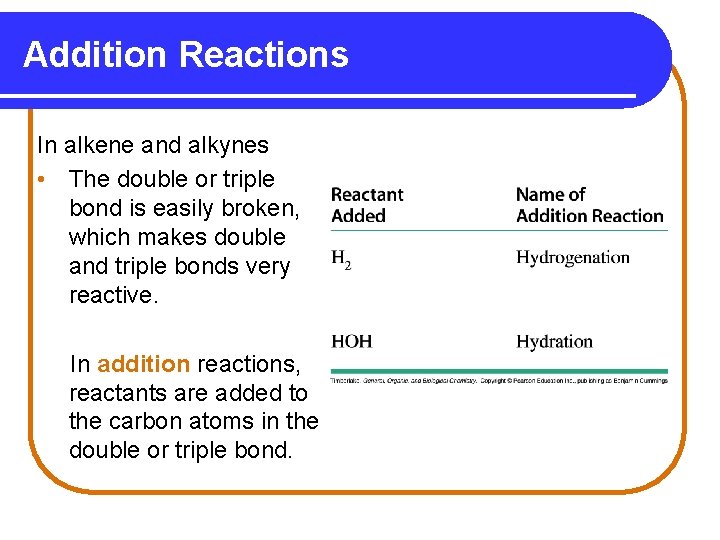
Addition Reactions In alkene and alkynes • The double or triple bond is easily broken, which makes double and triple bonds very reactive. In addition reactions, reactants are added to the carbon atoms in the double or triple bond.

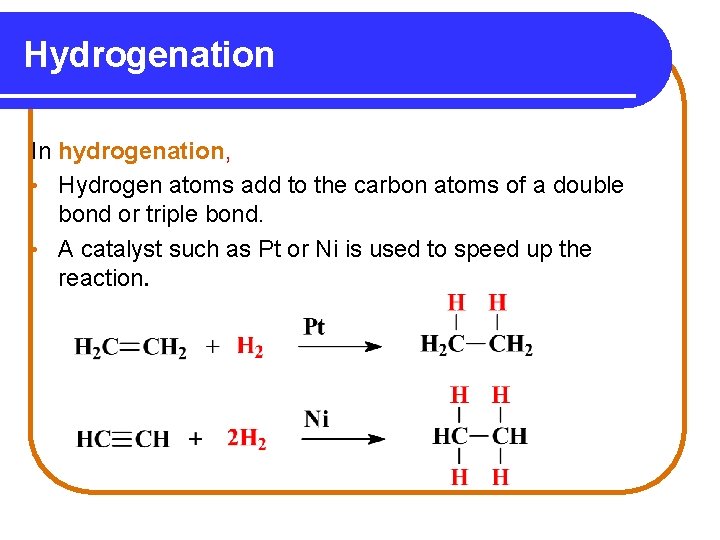
Hydrogenation In hydrogenation, • Hydrogen atoms add to the carbon atoms of a double bond or triple bond. • A catalyst such as Pt or Ni is used to speed up the reaction.

Hydrogenation of Oils Adding H 2 to double bonds in vegetable oils produces Compounds with higher melting points. • Solids at room temperature such as margarine, soft margarine, and shortening. •

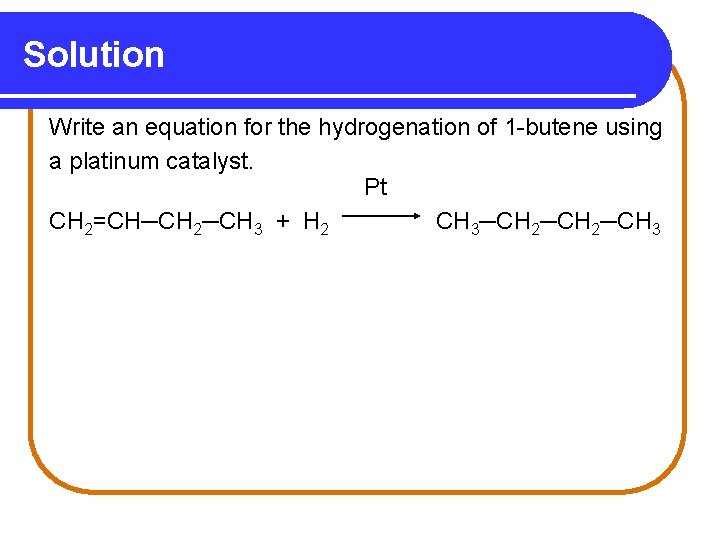
Learning Check Write an equation for the hydrogenation of 1 -butene using a platinum catalyst.

Solution Write an equation for the hydrogenation of 1 -butene using a platinum catalyst. Pt CH 2=CH─CH 2─CH 3 + H 2 CH 3─CH 2─CH 3

Trans Fats In vegetable oils, the unsaturated fats usually contain cis double bonds. • During hydrogenation, some cis double bonds are converted to trans double bonds (more stable) causing a change in the fatty acid structure. • If a label states “partially” or “fully hydrogenated”, the fats contain trans fatty acids.

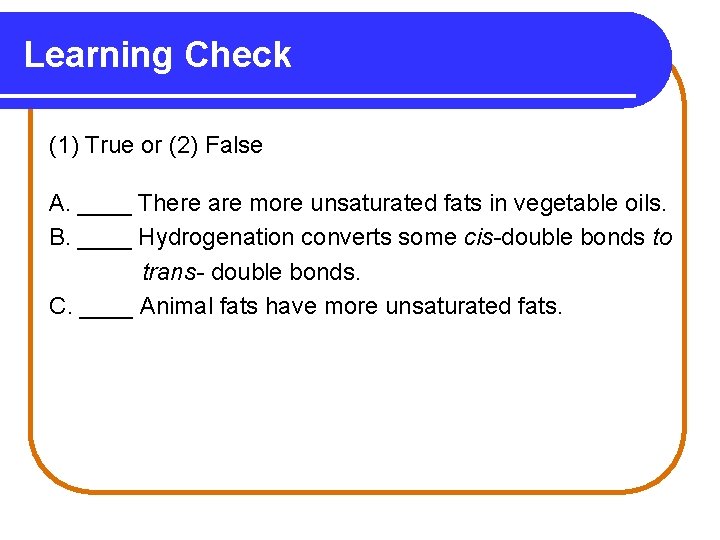
Learning Check (1) True or (2) False A. ____ There are more unsaturated fats in vegetable oils. B. ____ Hydrogenation converts some cis-double bonds to trans- double bonds. C. ____ Animal fats have more unsaturated fats.

Solution (1) True or (2) False A. 1 There are more unsaturated fats in vegetable oils. B. 1 Hydrogenation of oils converts some cis-double bonds to trans- double bonds. C. 2 Animal fats have more unsaturated fats.

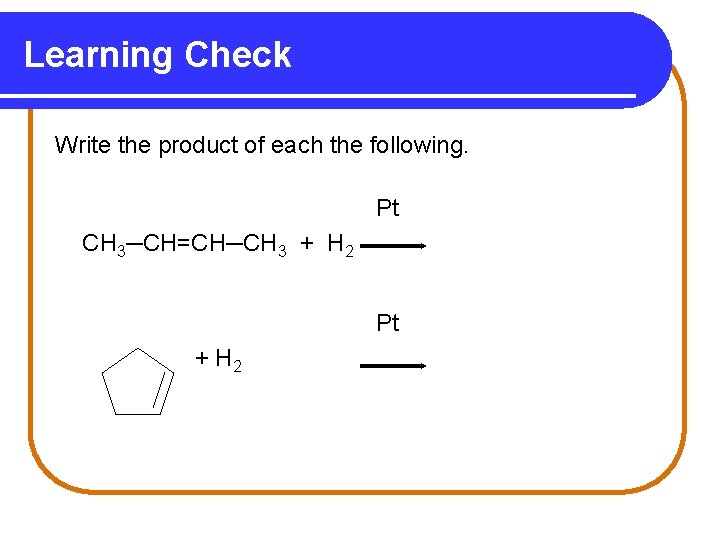
Learning Check Write the product of each the following. Pt CH 3─CH=CH─CH 3 + H 2 Pt + H 2

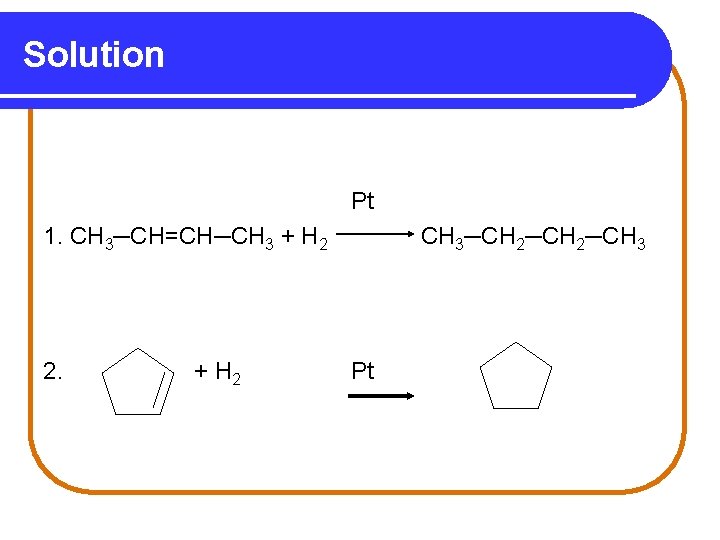
Solution Pt 1. CH 3─CH=CH─CH 3 + H 2 2. + H 2 CH 3─CH 2─CH 3 Pt

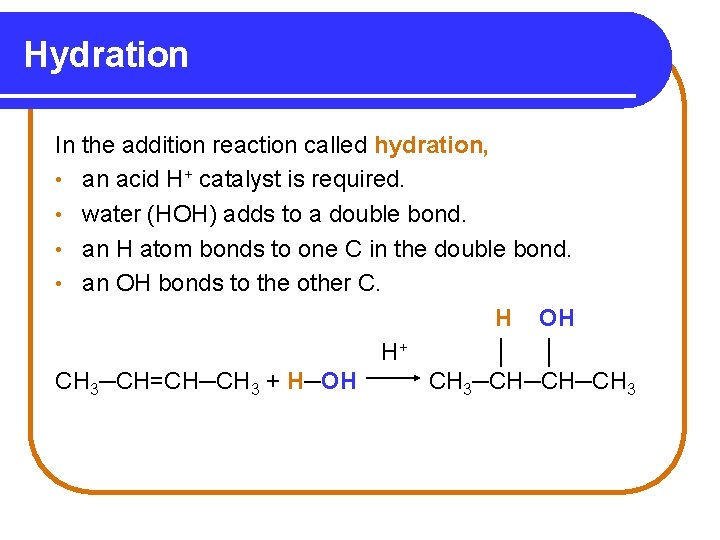
Hydration In the addition reaction called hydration, • an acid H+ catalyst is required. • water (HOH) adds to a double bond. • an H atom bonds to one C in the double bond. • an OH bonds to the other C. H OH H+ │ │ CH 3─CH=CH─CH 3 + H─OH CH 3─CH─CH─CH 3

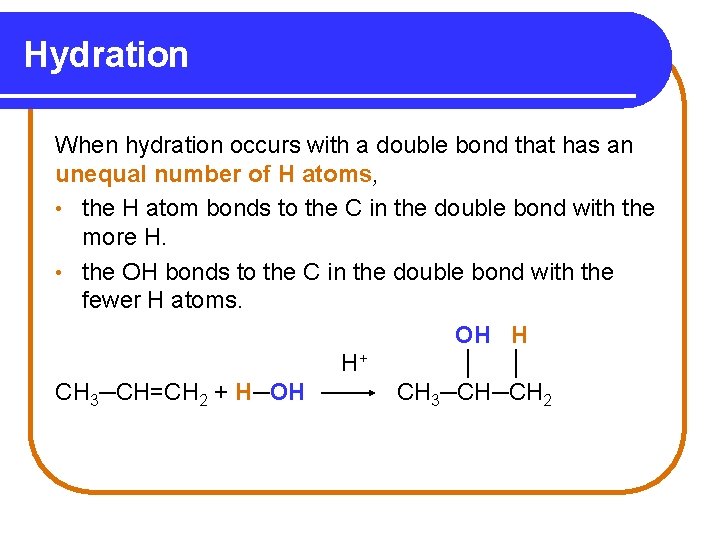
Hydration When hydration occurs with a double bond that has an unequal number of H atoms, • the H atom bonds to the C in the double bond with the more H. • the OH bonds to the C in the double bond with the fewer H atoms. OH H H+ │ │ CH 3─CH=CH 2 + H─OH CH 3─CH─CH 2

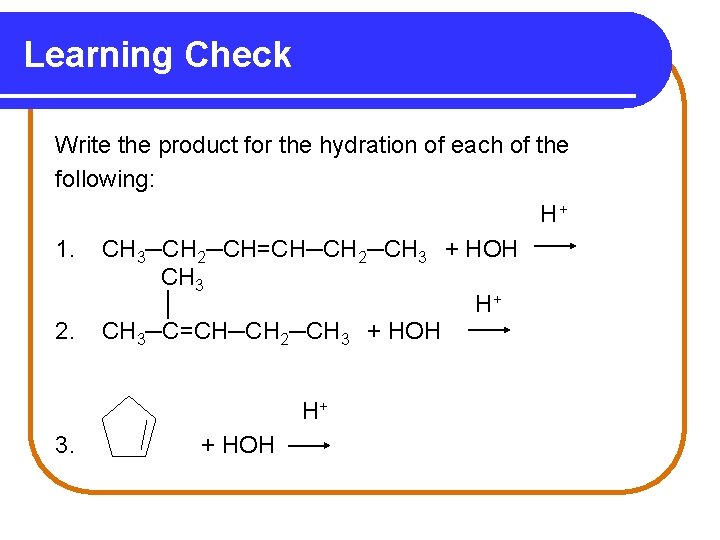
Learning Check Write the product for the hydration of each of the following: H+ 1. CH 3─CH 2─CH=CH─CH 2─CH 3 + HOH CH 3 │ H+ 2. CH 3─C=CH─CH 2─CH 3 + HOH H+ 3. + HOH

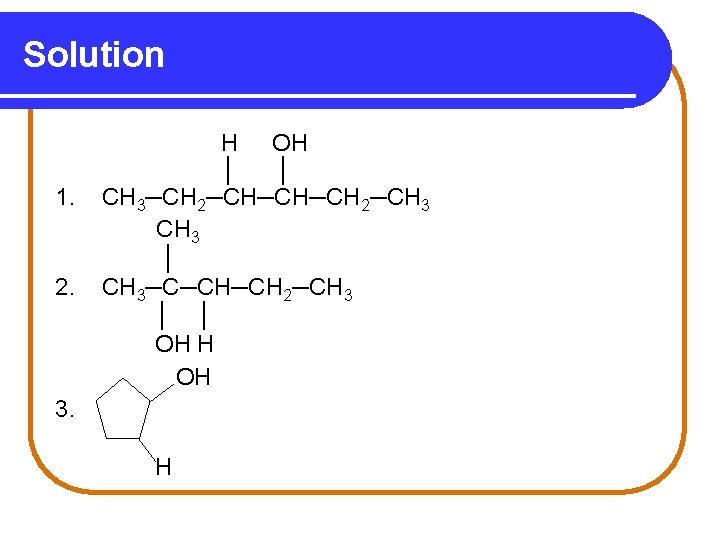
Solution 1. 2. H OH │ │ CH 3─CH 2─CH─CH─CH 2─CH 3 │ CH 3─C─CH─CH 2─CH 3 │ │ OH H OH 3. H